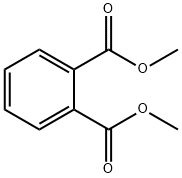
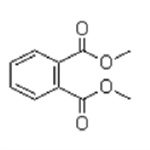
Dimethyl phthalate
Dimethyl phthalate esters is a broad-spectrum, highly effective insect repellent, it is colorless to pale yellow transparent liquid, is an effective insect repellent ingredient in toilet water, it has a good repellent effect on flies, lice, ants, mosquitoes, cockroaches, midges, gadfly, flat fleas, sand fleas, sand midges, sandflies, cicadas; its repellent effect lasts for a long time, and it can be used in different climatic conditions . Under the conditions of using, it’s chemical stable, displaying both high thermal stability and high resistance to sweat. It has good compatibility with common cosmetic and pharmaceutical agents, can be made of solutions, emulsions, pastes, coating agents, gel, aerosol, mosquito coils, micro-capsules and other special repellent agents, and also can be added to other products or materials (such as toilet water), so that product displays the repellent effect as well. Compared with standard mosquito repellent agent and mosquito repellent gel, it is less toxic, and less irritating, with longer repellent time and other notable features, is a repellent gel replacement.
The above information is edited by the chemicalbook of Tian Ye.
Toxicity
Dimethyl phthalate displays no toxicity or side effects on the skin and mucous membranes, non-allergic and non-skin-permeable etc., it is very safe to use, it is noted that people with sensitive skin may cause skin allergies reaction if repellent ester component is excessive absorbed .
Chemical Properties
Colorless oily liquid with a slightly aromatic fragrance. Immiscibility with ethanol, ethyl ether , soluble in organic solvents such as benzene, acetone, insoluble in water and mineral oil.
Uses
1. It is used as a plasticizer for cellulose acetate, polyvinyl fluoride coating repellent agents and solvents.
2. Dimethyl Phthalate is rodenticide rat poison, rat finished, chlorine mouse ketone intermediates, it is also an important solvent.
3. The product is a plasticizer for variety of resin having a very strong dissolving power, is compatible with a variety of cellulose resin, rubber, vinyl resin. It displays good film-forming character, adhesion and water resistance. It is usually used with diethyl phthalate in cellulose acetate film, varnish, transparent paper and molding powder production . A small amount of the product is used in nitrocellulose production. The product can also be used as a nitrile rubber plasticizers, with good resistance to cold products. The product can be mixed with other plasticizers. It can overcome the high volatility , low-temperature crystallization and other shortcomings. The products are also used as DEET oil (crude oil) and DDT solvents. The product is also used as gas chromatography stationary phase.
Production method
Esterified under normal pressure with an excess amount of the anhydride (4-fold) in methanol, excess methanol was refluxed with water, DMP was obtained from the reaction. The 600kg anhydride, 450kg methanol 90-95 ℃', 1050ml concentrated sulfuric acid were added into the reaction pot successively, heated to reflux for 24h. After completion of the reaction, methanol recovery, then neutralized with sodium carbonate, washed with water, and then distilled to obtain the finished product. Industrial grade product dimethyl phthalate purity of ≥99%. Material consumption fixed: anhydride 750kg/t, methanol 445kg/t.
Toxicity grading
Low toxicity
Acute toxicity
Oral-rat LD50: 6800 mg/kg; Oral-Mouse LD50: 6800 mg/kg
Irritation data
Eye-rabbit 119 mg
Flammability hazard characteristics
In the case of fire, high temperature, strong oxidants, it is combustible; combustion exhaust irritating smoke.
Storage feature
It should be stored in a complete package with care; warehouse ventilation, away from obvious flame, heat, and oxidants.
Extinguishing agents
Foam, carbon dioxide, dry powder, sand, water mist.
Professional Standards
TLV-TWA 5 mg/m³; STEL 10 mg/m3
Description
Dimethyl phthalate (DMP) is a member of the group of esters of phthalic acid commonly known as phthalates, used ubiquitously as solvents and plasticisers worldwide. Phthalates are plasticizers, and increase the flexibility of plastics. They are also found in deodorant formulations, perfumes, emollients and insect repellents.
Chemical Properties
Dimethyl phthalate occurs as a colorless, or faintly colored, odorless, viscous, oily liquid.
Physical properties
Clear, colorless, odorless, moderately viscous, oily liquid
History
Screened during World War II, this repellent is exceptionally effective against A. aegypti, lasting 196 d on cloth. Tests have been run against the newer pests A. albopictus and A. aegypti, including five repellents containing DEET (test standard), a controlled release formulation containing DEET, two dosages of DEET in ethanol, and Avon Skin-So- Soft. On the skin, the repellent chemicals provide significant protection from biting; however, A. albopictus is more sensitive to repellents than A. aegypti.
Uses
Dimethyl Phalate is the methyl ester of phthalic acid. Dimethyl phthalate is an ectoparasiticide and has many other uses, including in solid rocket propellants, plastics, and insect repellents.
Uses
Dimethyl phthalate is used as an insect repellant. It has also been employed as a solvent for cellulose acetate.
Uses
Solvent and plasticizer for cellulose acetate and cellulose acetate-butyrate compositions. Insect repellent for personal protection against biting insects.
Definition
ChEBI: Dimethyl phthalate is a phthalate ester, a diester and a methyl ester.
Production Methods
Dimethyl phthalate is produced industrially from phthalic anhydride and methanol.
General Description
Dimethyl phthalate appears as a water-white liquid without significant odor. Denser than water and insoluble in water. Hence sinks in water. Flash point 300 °F. Eye contact may produce severe irritation and direct skin contact may produce mild irritation. Used in the manufacture of a variety of products including plastics, insect repellents, safety glass, and lacquer coatings.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Dimethyl phthalate reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides. Can generate electrostatic charges by swirling or pouring [Handling Chemicals Safely, 1980. p. 250].
Health Hazard
Symptoms unlikely from any exposure.
Health Hazard
The acute toxicity of dimethyl phthalate intest animals was found to be very low.Ingestion may produce irritation of the gas trointestinal tract, somnolence, hypotension,and coma. The oral LD50 value in miceis 6800 mg/kg. Animal studies have shownthat administration of this compound causeddevelopmental toxicity, having effects on fer tility. Maternal toxicity in Sprague-Dawleyrats was observed at a dietary treatmentlevel of 5% (Field 1989). There was noeffect on average litter size, fetal bodyweight, or the incidence of skeletal or vis ceral malformations.
Fire Hazard
Dimethyl phthalate is combustible.
Pharmaceutical Applications
Dimethyl phthalate is used in pharmaceutical applications as a solvent and plasticizer for film-coatings such as hydroxypropyl methylcellulose, cellulose acetate and cellulose acetate–butyrate mixtures.
In addition to a number of industrial applications, dimethyl phthalate is also widely used as an insect repellent with topical preparations typically applied as a 40% cream or lotion; it has also been applied as a tent fabric treatment.
Contact allergens
Phthalates are plasticizers and increase the flexibility of plastics. They are also found in deodorant formulations, perfumes, emollients, and insect repellents.
Safety Profile
Moderately toxic by
ingestion and intraperitoneal routes. Mldly
toxic by inhalation. Experimental
teratogenic and reproductive effects.
Mutation data reported. An eye irritant. A
pesticide and insect repellent. Combustible
when exposed to heat or flame; can react
with oxidizing materials. To fight fire, use
CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also ESTERS.
Safety
In pharmaceutical applications, dimethyl phthalate is used in film coating and as a topically applied insect repellent.Acute exposure to the eyes and mucous membranes can cause irritation, although dimethyl phthalate is considered less irritant than diethyl phthalate. Inhalation of dimethyl phthalate can cause irritation of the respiratory tract; oral ingestion can cause a burning sensation in the mouth, vomiting, and diarrhea. Owing to the low water solubility and relatively high lipid solubility, dimethyl phthalate may accumulate in body tissues after chronic exposure, which may cause central nervous system depression.
Although some animal studies have suggested that high concentrations of dimethyl phthalate may be teratogenic or cause mutagenic effects with bacteria,(5,6) other studies have shown no adverse effects.(7) There are no confirmed reports of human reproductive or developmental effects, and the compound is not generally regarded as a carcinogenic material.
LD50 (chicken, oral): 8.5g/kg
LD50 (guinea pig, oral): 2.4g/kg
LD50 (mouse, IP): 1.38g/kg
LD50 (mouse, oral): 6.8g/kg
LD50 (rabbit, oral): 4.40g/kg
LD50 (rat, IP): 3.38g/kg
LD50 (rat, oral): 6.80g/kg
Potential Exposure
AgriculturalChemical; Mutagen; Reproductive Effector; PrimaryIrritant. Dimethyl phthalate is used as a solvent, dye carrier,plasticizer for cellulose ester plastics, and as an insectrepellent.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
May leach from plastic products (e.g., tubing, containers) used in laboratories during
chemical analysis of aqueous samples.
Environmental Fate
Biological. In anaerobic sludge, degradation occurred as follows: monomethyl phthalate to phthalic acid to protocatechuic acid followed by ring cleavage and mineralization (Shelton et al., 1984). In a static-culture-?ask screening test, dimethyl phthalate showed significant biodegradation with rapid adaptation. The ester (5 and 10 mg/L) was statically incubated in the dark at 25°C with yeast extract and settled domestic wastewater inoculum. After 7 days, 100% biodegrada-tion was achieved (Tabak et al., 1981).
Photolytic. An aqueous solution containing titanium dioxide and subjected to UV light (λ >290 nm) yielded mono- and dihydroxyphthalates as intermediates (Hustert and Moza, 1988).
Chemical/Physical. Hydrolyzes in water forming phthalic acid and methyl alcohol (Wolfe et al., 1980).
storage
Dimethyl phthalate is sensitive to prolonged exposure to light and it should therefore be stored in a cool, dark, dry, well-ventilated area that is protected from physical damage, and isolated from incompatible substances. Containers of dimethyl phthalate may be hazardous when empty as they may retain product residues such as vapors and liquids. There is a slight fire hazard when exposed to heat, and above the flash point explosive vapor–air mixtures may be formed.Carbon dioxide and carbon monoxide are released when dimethyl phthalate is heated to decomposition. Solutions of dimethyl phthalate in acetone, dimethyl sulfoxide, ethanol (95%), and water are stable for 24 hours under normal laboratory conditions.
Shipping
The name of this material is not in the DOT listof materials for label and packaging standards. However,based on regulations, it may be classified as anEnvironmentally hazardous substances, liquid, n.o.s. It fallsin Hazard Class 9 and Packing Group III.[20,
Incompatibilities
Dimethyl phthalate is incompatible with strong acids or bases, nitrates, and strong oxidizing agents. As with other phthalates, contact with plastics should be avoided.
Toxics Screening Level
The Initial Threshold Screening Level (ITSL) for Dimethyl Phthalate is 50 μg/m3 based on an 8 hour averaging time.
Regulatory Status
Dimethyl phthalate is included in a number of topical pharmaceutical formulations. Included in the FDA Inactive Ingredients Database (oral tablets, sustained action). As from 1992, dimethyl phthalate is no longer registered for use as a pesticide in California.