Description
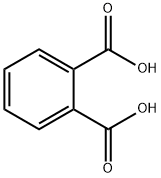
Phthalic acid is an aromatic dicarboxylic acid, with formula C
6H
4(CO
2H)
2. It is an isomer of isophthalic acid and terephthalic acid. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale.
Chemical Properties
PHTHALIC ACID,C6H4(COOH)2, mp 208 °C (ortho), 330 °C (meta and iso), the ortho form sublimes and the meta and iso forms decompose with heat, sp gr 1.593 (ortho). Phthalic acid is very slightly soluble in H2O, soluble in alcohol, and slightly soluble in ether. The solid form is colorless, crystalline
Uses
It is a dibasic acid, with pKa's of 2.89 and 5.51. The mono potassium salt, potassium hydrogen phthalate is a standard acid in analytical chemistry. Typically phthalate esters are prepared from the widely available phthalic anhydride. Reduction of phthalic acid with sodium amalgam in the presence of water gives the 1,3- cyclohexadiene derivative.
Uses
Phthalic Acid (Phenyl-13C6, D4) is labelled Phthalic Acid (P384480) which is an organic reagent used to synthesize phthalates.
Definition
ChEBI: A benzenedicarboxylic acid cosisting of two carboxy groups at ortho positions.
Production Methods
Phthalic acid is produced by the catalytic oxidation of naphthalene directly to phthalic anhydride and a subsequent hydrolysis of the anhydride.
Phthalic acid was first obtained by French chemist Auguste Laurent in 1836 by oxidizing naphthalene tetrachloride. Believing the resulting substance to be a naphthalene derivative, he named it "naphthalic acid". After the Swiss chemist Jean Charles Galissard de Marignac determined its correct formula, Laurent gave it its present name. Manufacturing methods in the nineteenth century included oxidation of naphthalene tetrachloride with nitric acid, or, better, oxidation of the hydrocarbon with fuming sulfuric acid, using mercury or mercury(II) sulfate as a catalyst.
Definition
phthalic acid: colourlesscrystalline dicarboxylic acid,C
6H
4(COOH)
2; r.d. 1.6; m.p. 207°C.The two –COOH groups are substitutedon adjacent carbon atoms ofthe ring, the technical name beingbenzene-1,2-dicarboxylic acid. Theacid is made from phthalic anhydride(benzene-1,2-dicarboxylic anhydride,C
8H
4O
3), which is made by the catalyticoxidation of naphthalene. Theanhydride is used in making plasticizersand polyester resins.
Synthesis Reference(s)
The Journal of Organic Chemistry, 30, p. 2414, 1965
DOI: 10.1021/jo01018a074
General Description
White crystals or fine white powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phthalic acid is a carboxylic acid. Phthalic acid is sensitive to exposure to extreme heat. Phthalic acid reacts violently with nitric acid. Phthalic acid is incompatible with sodium nitrite. Phthalic acid is also incompatible with oxidizers. .
Fire Hazard
Phthalic acid is combustible.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by
ingestion and intraperitoneal routes. A skin
and mucous membrane irritant.
Combustible when heated. In the form of
dust (anhydride) it can explode. Mixtures
with sodmm nitrite explode when heated.
Violent reaction with HNO3. When heated
to decomposition it emits acrid smoke and
irritating fumes. Used in synthesis of dyes
and dyestuffs, in medcines and perfumes.
Safety
The toxicity of phthalic acid is low with LD
50 (mouse) of 550 mg/kg. However, many phthalate esters have been implicated as endocrine disruptors.
Purification Methods
Crystallise phthalic acid from water. [Beilstein 9 IV 3167.]
Isomers
Phthalic acid is one of three isomers of benzene dicarboxylic acid, the others being iso phthalic acid and terephthalic acid. Sometimes the term "phthalic acids" is used to refer to this family of isomers, but in the singular, "phthalic acid", refers exclusively to the ortho- isomer.