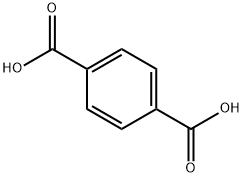
Description
Terephthalic acid is the organic compound with formula C
6H
4(COOH)
2. This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tones are produced annually. It is one of three isomeric phthalic acids.
Chemical Properties
Terephthalic acid is a white crystalline solid. Soluble in alkaline solution, slightly soluble in hot ethanol, insoluble in water, ether, glacial acetic acid and chloroform, consequently up until around 1970 most crude terephthalic acid was converted to the dimethyl ester for purification. It sublimates when heated.
History
Terephthalic acid came to prominence through the work of Winfield and Dickson in Britain around 1940. Earlier work by Carothers and coworkers in the United States established the feasibility of producing high molecular weight linear polyesters by reacting diacids with diols, but they used aliphatic diacids and diols. These made polyesters which were unsuitable to be spun into fibers. Winfield and Dickson found that symmetrical aromatic diacids yield high-melting, crystalline, and fiberforming materials; poly(ethylene terephthalate) (PET) has since become the largest volume synthetic fiber.
Uses
Terephthalic acid (TPA) is a benzenepolycarboxylic acid with potential anti-hemorrhagic properties. It is a high-tonnage chemical, widely used in the production of synthetic materials, notably polyester fibers (poly-(ethylene terephthalate)).
Application
1,4-benzenedicarboxylic acid is mainly used for the production of poly (ethylene terephthalate). Also production of plasticizer dioctyl phthalate (DOTP) and polyester plasticized agents. 1,4-benzenedicarboxylic acid and polyhydric alcohols have a condensation reaction withd iethylene glycol, triethylene glycol, glycerol, propylene glycol, butylene glycol, etc. preparation of the polyester plasticizer.
Definition
ChEBI: Terephthalic acid is a benzenedicarboxylic acid carrying carboxy groups at positions 1 and 4. One of three possible isomers of benzenedicarboxylic acid, the others being phthalic and isophthalic acids. It is a conjugate acid of a terephthalate(1-).
Application
Virtually the entire world's supply of terephthalic acid and dimethyl terephthalate are consumed as precursors to polyethylene terephthalate (PET). World production in 1970 was around 1.75 million tones. By 2006, global purified terephthalic acid (PTA) demand had exceeded 30 million tonnes.
There is a smaller, but nevertheless significant, demand for terephthalic acid in the production of poly butylene terephthalate and several other engineering polymers.
Production Methods
Terephthalic acid is produced by oxidation of p-xylene by oxygen in air:
This reaction proceeds through a p-toluic acid intermediate which is then oxidized to terephthalic acid. In p-toluic acid, deactivation of the methyl by the electron withdrawing carboxylic acid group makes the methyl one tenth as reactive as xylene itself, making the second oxidation significantly more difficult . The commercial process utilizes acetic acid as solvent and a catalyst composed of cobalt and manganese salts, with a bromide promoter.
Preparation
The major commercial route to terephthalic acid which is suitable for the
direct preparation of poly(ethylene terephthalate) is from p-xylene:
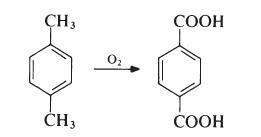
p-Xylene is obtained largely from petroleum sources, being a product of the
fractionation of reformed naphthas. The oxidation is carried
out in the liquid phase. Typically, air is passed into a solution of p-xylene in
acetic acid at about 200?? and 2 MPa (20 atmospheres) in the presence of a
catalyst system containing cobalt and manganese salts and a source of
bromide ions. The terephthalic acid produced contains only small amounts of
impurities (mainly p-carboxybenzaldehyde), which are readily removed. The
acid is dissolved in water at about 2500 e and 5 MPa (50 atmospheres) and
treated with hydrogen (which converts the aldehyde to p-toluic acid). The
solution is then cooled to 100?? and pure terephthalic acid crystallizes.
Synthesis
Benzoic acid, phthalic acid and other benzene-carboxylic acids in the form of alkali-metal salts, comprise the chargestock. In a first step, the alkali-metal salts (usually potassium) are converted to terephthalates when heated to a temperature exceeding 350 °C (662 °F). The dried potassium salts (of benzoic acid or o- or isophthalic acid) are heated in anhydrous form to approximately 420 °C (788 °F) in an inert atmosphere (CO2) and in the presence of a catalyst (usually cadmium benzoate, phthalate, oxide, or carbonate). The corresponding zinc compounds also have been used as catalysts. In a following step, the reaction products are dissolved in H2O and the terephthalic acid precipitated out with dilute H2SO4. The yield of terephthalic acid ranges from 95 to 98%.
Synthesis Reference(s)
Chemistry Letters, 15, p. 299, 1986
Journal of the American Chemical Society, 82, p. 2876, 1960
DOI: 10.1021/ja01496a051The Journal of Organic Chemistry, 44, p. 4727, 1979
DOI: 10.1021/jo00393a063
General Description
White powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Terephthalic acid is a carboxylic acid. Terephthalic acid donates hydrogen ions if a base is present to accept them. This "neutralization" generates substantial amounts of heat and produces water plus a salt. Insoluble in water but even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Terephthalic acid to corrode or dissolve iron, steel, and aluminum parts and containers. May react with cyanide salts to generate gaseous hydrogen cyanide. Will react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by reaction with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. React with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization reactions; may catalyze (increase the rate of) chemical reactions.
Fire Hazard
Flash point data for Terephthalic acid are not available. Terephthalic acid is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by intravenous and intraperitoneal routes. Mildly toxic by ingestion. An eye irritant, Can explode during preparation. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
TPA is used primarily in the production of polyethylene terephthalate polymer for the fabrication of polyester fibers and films. A high-volume production chemical in the United States.
Purification Methods
Purify the acid via the sodium salt which, after crystallisation from water, is re-converted to the acid by acidification with mineral acid. Filter off the solid, wash it with H2O and dry it in a vacuum. The S-benzylisothiuronium salt has m 204o (from aqueous EtOH). [Beilstein 9 IV 3301.]
Incompatibilities
Combustible; dust may form an explosive mixture with air. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.