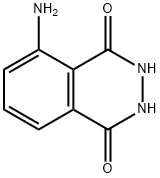
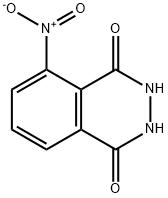
Chemical fluorescent molecules
Luminol is an organic compound which, when oxidized, emits light — a phenomenon known as chemiluminescence. This is similar to the reactions that fireflies uses to emit light, and to those used in "glow-sticks" and some roadside emergency lights. In this reaction, a small amount of luminol (3-aminophthalhydrazide or 5-amino-2,3-dihydro- 1,4-phthalazinedione) is dissolved in a basic aqueous solution, which also contains a small amount of copper(II) sulfate. To this solution is added a solution of a mild oxidizing agent, which is 0.3% hydrogen peroxide in the demonstration below. (Bleach is also used in some recipes as the oxidizing agent.) The reaction is believed to occur by the following mechanism:
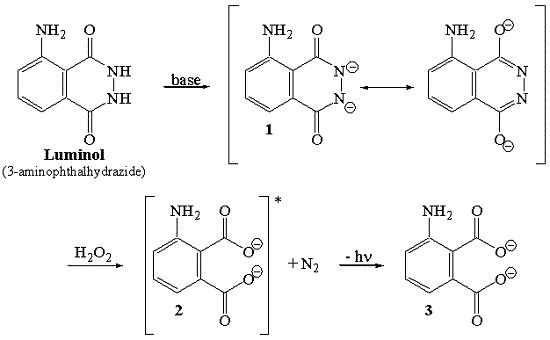
Chemiluminescence method
Luminol chemiluminescence has been trialed for estimating the PMI from skeletal remains. Luminol methods are based on the reaction between luminol and hydrogen peroxide which is catalyzed by iron in hemoglobin found in fluids and tissues from bodies. This reaction results in a chemiluminescence light which can be measured by various means .
Chemical properties
Yellow crystalline powder, Easily soluble in lye, soluble in dilute acid but almost insoluble in water and hardly soluble in alcohol. When its neutral or weak acidic solution is exposed to ultraviolet light, it exhibits strongly bright blue fluorescence. Melting point: 329-332 ° C
Uses
1. It can be used as chemical analysis reagents, indicators.
2. It can be used for chemiluminescence analysis such as measurement of metal cations, blood and glucocorticoids
Hazards & Safety Information
Category Toxic substances
Toxic grading poisoning
Acute toxicity Oral – rat; LD50: > 500 mg/kg
Flammability and Hazardous properties it is combustible with combustion producing toxic nitrogen oxide fumes
Storage and transportation characteristics warehouse: ventilated, low temperature and dry
Fire extinguishing agent dry powder, foam, sand, carbon dioxide, mist water
Chemical Properties
yellow crystals or beige powder
Uses
Luminol is a chemiluminescent probe that has been used to detect myeloperoxidase-mediated oxidative events in granulocytes and for chemiluminescence analysis of metal cations and blood.
Uses
Luminol is used in the detection of copper, iron, peroxides and cyanides. It exhibits chemiluminescence and utilized to measure opsonic and phagocytic function. It acts as a biological sensor and involved in the detection of polymorphonuclear leukocytes response in a patient with a myeloperoxidase deficiency. Further, it is used as a forensic test for blood.
Uses
Detection of copper, iron, peroxides, cyanides.
General Description
Yellow crystals or light beige powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Oxidation of 3-Aminophthalhydrazide is accompanied by a striking emission of light. . .
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 3-Aminophthalhydrazide emits toxic fumes.
Fire Hazard
Flash point data for 3-Aminophthalhydrazide are not available, but 3-Aminophthalhydrazide is probably combustible.
Synthesis
The synthesis of Luminol is as follows: To a reaction tube, add 140 mg of 3-nitrophthalhydrazide and 1.0 mL of 3 M sodium hydroxide solution. Stir with a rod, and to the resulting deep brown-red solution add 0.6 g of sodium hydrosulfite dihydrate (Na2S2O4 · 2 H2O, MW 210.2). Wash down the sides of the tube with a small amount of water. Heat to a gentle boil and keep the tube hot for 5 minutes. During this time some product may begin to crystallize. Add 0.4 mL of acetic acid, cool the tube in cold water, and stir. Collect the light-yellow product Luminol by suction filtration.
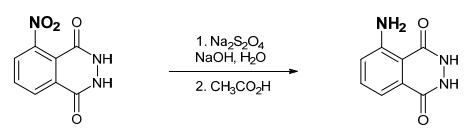
Purification Methods
Dissolve luminol in KOH solution, treat with Norit (charcoal), filter and precipitate it with conc HCl. [Hardy et al. Talanta 24 297 1977.] Store it in the dark in an inert atmosphere, because its structure changes during its luminescence. It has been recrystallised from 0.1M KOH [Merenyi et al. J Am Chem Soc 108 77716 1986]. [Beilstein 25 II 389, 25 III/IV 4192.]