How to synthesize Terephthalic acid?
Description
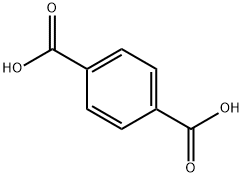
Purified terephthalic acid (PTA) is a white, crystalline solid with negligible vapor pressure under standard conditions. The IUPAC name is Benzene-1,4-dicarboxylic acid. PTA is one of the largest-volume commodity chemicals. Nearly all PTA is consumed in polyester production. It is predominantly used for producing saturated polyesters, mainly polyethylene terephthalate (PET), and related polymer products such as fibers, resins, thin films, bottles, etc. A small portion of terephthalic acid is used to produce semi-aromatic and aromatic polyamides and intermediate chemicals such as cyclohexanedimethanol, terephthaloyl chloride, liquid crystal polymer, etc.
Solubility
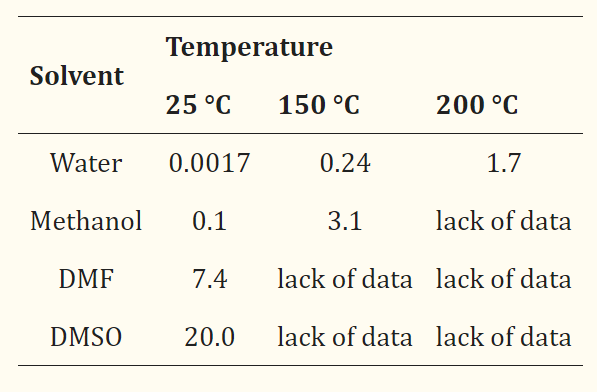
Terephthalic acid is poorly soluble in organic solvents (Table 1). Among all tested solvents, the best solubility of PTA was observed in DMSO (20 g of PTA per 100 g DMSO at 25 °C). PTA is also soluble in N-methyl-2-pyrolidone and dimethylamine, but the solubility at 90 °C is two times lower than in DMSO. In general, the solubility of PTA in organic solvents is low and slowly increases with increasing temperature[1].
Production method
Terephthalic acid (p-TA or TA), a raw material for polyethylene terephthalate (PET) production, is one of the most essential chemicals in the petrochemical industry. Crude terephthalic acid (CTA), commonly produced by homogeneous liquid phase p-xylene oxidation, contains impurities such as 4-carboxybenzaldehyde (4-CBA, 2000-5000 ppm) and several colored polyaromatics that should be removed to obtain purified terephthalic acid (PTA). PTA is manufactured by hydropurification of CTA over carbon-supported palladium catalyst (Pd/C) in the current industry[2].
Usually, PTA is produced in a two-stage process. In the first stage, crude terephthalic acid (CTA) is produced by liquid-phase oxidation of para-xylene (p-xylene) in acetic acid medium in the presence of a cobalt acetate and manganese acetate catalyst with hydrogen bromide as a source of bromine promotor. Typically, CTA contains impurities such as 4-carboxybenzaldehyde (4-CBA), p-tolualdehyde, benzoic acid, and p-toluic acid, which are responsible for changing the final product's color. In the second stage, CTA produced by the oxidation reaction is then purified through hydrogenation. Purification of CTA typically requires at least one chemical transformation (e.g., hydrogenation) in addition to physical procedures such as crystallization, filtration, and drying to produce the final PTA product. Purification of PTA is necessary to achieve high-purity and high-value products.
References
[1] Karolina Matuszek. “Studies on the Solubility of Terephthalic Acid in Ionic Liquids.” Molecules 25 1 (2019).
[2] Jinghong Zhou. “Terephthalic acid hydropurification over Pd/C catalyst.” Studies in Surface Science and Catalysis 51 1 (2006): 293–296.
You may like
Related articles And Qustion
See also
Lastest Price from Terephthalic acid manufacturers

US $0.00-0.00/KG2025-07-16
- CAS:
- 100-21-0
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/KG2025-04-21
- CAS:
- 100-21-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt