The Synthesis method and Toxicity of 18-Crown-6
Description
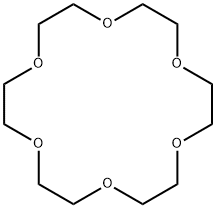
The compound known as 18-crown-6 is one of the simplest and most valuable of the macrocyclic polyethers. Pedersen first reported its synthesis in low yield. Greene, Dale and Kristiansen have reported syntheses of the title compound from triethylene glycol and triethylene glycol di-p-toluenesulfonate. Both of these procedures use strong base and anhydrous conditions and achieve purification by more or less classical methods. The combination of distillation and formation of the acetonitrile complex affords a crown of high purity without lengthy chromatography or sublimation.
Synthesis method
A 3-l., three-necked flask equipped with a mechanical stirrer, a reflux condenser, and an addition funnel is charged with 112.5 g (100.0 ml., 0.7492 mol) of trimethylene glycol and 600 ml of tetrahydrofuran. Stirring is begun, and a 60% potassium hydroxide solution, prepared by dissolving 109 g (1.65 mol) of 85% potassium hydroxide in 70 ml water, is added. The solution warms slightly. After about 15 minutes of vigorous stirring (the solution begins to develop color and gradually becomes rust brown), a solution of 140.3 g (0.7503 mol) of 1,2-bis(2-chloroethoxy)ethane in 100 ml of tetrahydrofuran is added in a stream. After the addition is complete, the solution is heated at reflux and stirred vigorously for 18–24 hours. The solution is allowed to cool, and the bulk of the tetrahydrofuran is evaporated under reduced pressure. The resulting thick, brown slurry is diluted with 500 ml of dichloromethane and filtered through a glass frit. The salts removed by filtration are washed with more dichloromethane to remove the absorbed crown, and the combined organic solution is dried over anhydrous magnesium sulfate, filtered, evaporated to minimum volume (aspirator vacuum), and distilled under high vacuum using a simple distillation head. The distillation should be carried out at the lowest possible pressure; a typical fraction contains 76–87 g (38–44%) of crude 18-crown-6 and is collected over 100–167° (0.2 mm).
To 50 g of the crude 18-crown-6 in a 250-ml Erlenmeyer flask, 100 ml of acetonitrile is added. A magnetic stirring bar is added, and the flask is equipped with a calcium chloride drying tube. The resulting slurry is heated on a hot plate to affect the solution. The solution is stirred vigorously as it is allowed to cool to ambient temperature; fine white crystals of the crown-acetonitrile complex are deposited. The flask is allowed to stand in a freezer for 24–48 hours and is finally cooled in a −30° bath to precipitate as much of the complex as possible. The solid is collected by rapid filtration and washed once with a small amount of cold acetonitrile. The hygroscopic crystals are transferred to a 200-ml, round-bottomed flask with a magnetic stirring bar and a vacuum takeoff. The acetonitrile is removed from the complex under high vacuum (0.1–0.5 mm), with gentle heating (~35°), over 2–3 hours. The pure colorless 18-Crown-6(28–33 g, 56–66%) crystallizes on standing, m.p. 38–39.5°.
Toxicity
18-CROWN-6 was assessed for neurologic effects in rats, mice, and rabbits by intravenous (IV) and intraperitoneal (IP) routes of administration. Male rats and mice exhibited no effects with 20 mg/kg IV doses. Given IP doses of 20 to 160 mg/kg/day, rats and mice exhibited numerous signs, including aggression, tremors, muscle weakness, and degradation of some reflexes. All signs faded after four days when dosage levels were kept constant but returned when the dose was doubled. All signs disappeared upon discontinuance of exposure. Treatment with PCPA (p-chlorophenylalanine) or dibenzyline caused most signs to disappear. Rabbits given 6.0 mg/kg/day IV displayed tremors, hyperactivity, unsteady gait, and stereotypic behavior, with acclimation as in rats and mice. 18-CROWN-6 had no activity in isolated tissue preparations unless it was first incubated with tissues. PCPA and dibenzyline reversibly blocked the actions of incubated 18-CROWN-6 on isolated tissue[1-2]. 18-CROWN-6 is hypothesized to be metabolized to a serotonergic agonist.
References
[1] Richard R. Hendrixson . “Oral toxicity of the cyclic polyethers—12-crown-4, 15-crown-5, and 18-crown-6—in mice.” Toxicology and applied pharmacology 44 2 (1978): Pages 263-268.
[2] S C Gad. “Behavioral and neuropharmacological toxicology of the macrocyclic ether 18-crown-6.” Drug and Chemical Toxicology 1 4 (1978): 339–53.
You may like
Related articles And Qustion
See also
Lastest Price from 18-Crown-6 manufacturers

US $0.00/kg2025-09-01
- CAS:
- 17455-13-9
- Min. Order:
- 100kg
- Purity:
- 99%
- Supply Ability:
- 50 MT

US $0.00/KG2025-04-21
- CAS:
- 17455-13-9
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 30tons/month