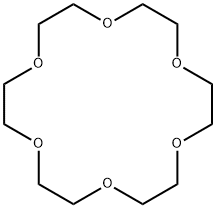
18-Crown-6 - Reaction / Application on Synthetic Works
18-crown-6 (18C6, 18c6, [18]-Crown-6) functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1) [1]. It can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst.[2][3] It can also be used as a metal complexing agent to prepare a variety of molecular complexes.[4]
The following example is about its application on the synthesis of alkali metal complexes derived from 1-phenyl-tetrazole-thiolate and crown ethers [1]
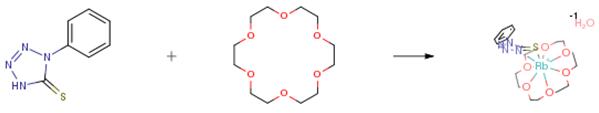
RbOH (0.17 g, 1.69 mmol) was added to a solution of 1-phenyl-1-H-tetrazole -5-thione (0.30 g, 1.69 mmol) in methanol (40 mL) at ambient temperature. The solution was stirred for 2 h and then 15-crown-5 ether (1,4,7,10,13- Pentaoxacyclopentadecane) (0.62 g, 2.81 mmol) was added. The reaction mixture was stirred for 5 h and the volume of the solution was reduced and allowed to crystallize. Yield: 88 percent. Mp: 147-149 °C
The following example is about its application on the synthesis of the silylated benzene radical anion salts [2]
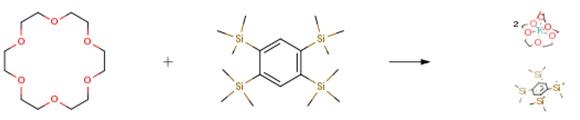
A solution of C6H2(SiMe3)4-1,2,4,5 (0.734 g, 2.00 mmol) and [18]crown-6 (1.145 g, 4.33 mmol) in toluene (100 cm3) was introduced into a Schlenk flask containing a K mirror (0.156 g, 4.00 mmol) at ambient temperature. The mixture was stirred vigorously for 2 d (the flask was tilted periodically from one side to the other to ensure that the stirring bar moved all over the flask, clearing the potassium surface from the precipitate). When all the potassium had disappeared, the dark brown precipitate was filtered off, washed with toluene and dried in a vacuum yielding the intermediate (1.850 g, 95percent). A sample of the intermediate (0.200 g, 0.205 mmol) in hexane (10 cm3) was treated with dry air (until the dark colour disappeared) and then with water (10 cm3). Evaporation of the hexane layer produced C6H2(SiMe3)4-1,2,4,5 (0.072 g, 0.196 mmol) and titration of the water layer with 0.1 M HCl revealed the presence of 0.40 mmol of KOH, thus confirming the 1:2 stoichiometry. The remaining compound 2 was dissolved in THF (30 cm3), layered with Et2O and stored at -10 °C; after several days a mixture of dark brown blocks and needles and a colorless microcrystalline powder was obtained.
The following example is about its application on the synthesis of [Mn]-hydrogenase. [3]
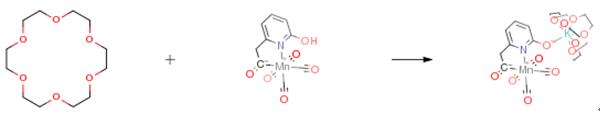
To a solution of the starting material (1.0 g,3.3 mmol) in THF (5 ml) was added KH (132 mg, 3.3 mmol) slowly under stirring at room temperature. When no more H2 gas formed, a THF solution of 18-crown-6 (958 mg, 3.6 mmol) was added at once to the mixture. The resulting mixture was further stirred at room temperature for 2 h before it was filtered through a Teflon membrane to remove all the solid impurity. A layer of Et2O was then added on top of the THF solution and the mixture was stored at -22 °C. A crystal of the product (18-crown-6) was obtained in 75% yield (1.5 g).
The following example is about its application on the synthesis of an inorganic–organic hybrid compound [4]
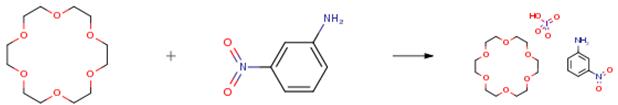
The product was prepared through the slow evaporation of a mixture containing HIO4 (50mg), 3-nitroaniline (20mg), and 18-crown-6 (200mg) in methanol (50mL), as shown in the picture. The methanol solution was allowed to stand for approximately five days under r.t. conditions. The single crystals of the product were yellow block crystals obtained with a 54 percent yield. The chemical formulas of the product were determined using elemental and X-ray crystallographic analyses. Anal. Calcd. C37H66IN4O25 for salt 1: C, 36.37percent; H, 5.26percent; N, 4.71percent. Found: C, 36.28percent; H, 5.13percent; N, 4.62 percent.
References
1.Hernández-Arganis M, Moya-Cabrera M, Jancik V, Martínez-Otero D, María Cotero-Villegas A, del Carmen Pérez-Redondo M, Cea-Olivares R. Synthesis and structural study of alkali metal complexes derived from 1-phenyl-tetrazole-thiolate and crown ethers[J]. Inorganica Chimica Acta, 2018, 475:83-89.
2.Hitchcock PB, Lappert MF, Protchenko AV. Synthesis and structure of the silylated benzene radical anion salts [K([18]crown-6){C6H4(SiMe3)2-1,4}] and [K([18]crown-6)(THF)2][C6H2(SiMe 3)4-1,2,4,5][J]. Journal of Organometallic Chemistry, 2011, 696(10):2161-2164.
3.Pan HJ, Huang G, Wodrich MD, Tirani FF, Ataka K, Shima S, Hu X. A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor[J]. Nature Chemistry, 2019, 11(7):669-675.
4.Liu ZQ, Liu Y, Chen Y, Zhao WQ, Fang WN. Synthesis, characterization, and phase transition of an inorganic–organic hybrid compound, [(3-nitroanilinium+) (18-crown-6)][IO4−](CH3OH)[J]. Chinese Chemical Letters, 2017, 28(2):297-301
You may like
Related articles And Qustion
Lastest Price from 18-Crown-6 manufacturers

US $0.00/kg2025-09-01
- CAS:
- 17455-13-9
- Min. Order:
- 100kg
- Purity:
- 99%
- Supply Ability:
- 50 MT

US $0.00/KG2025-04-21
- CAS:
- 17455-13-9
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 30tons/month