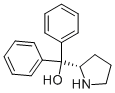
(S)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol- Reaction / Application on synthetic works
(S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol (DPPM) is a bifunctional organocatalyst[1], which could be used in the following processes:
- DPPM reacts with catecholborane to form a spiroborate ester, which can be an efficient catalyst for the synthesis of enantiopure alcohols by borane reduction of acetophenone.[2]
- The catalyst generated in situ by reacting DPPM with borane-diethylaniline, can efficiently catalyze the enantioselective reduction of 2′-fluoroacetophenone.[3]
- Mesoporous SBA-15 silica functionalized with DPPM can catalyze the addition of diethylzinc to benzaldehyde to form (S)-1-phenyl-propanol.
- Used to prepare the corresponding oxazaborolidines for the borane-mediated asymmetric reduction of ketones. [4]
The following example is about the catalytic function of DPPM [5]
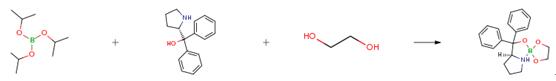
To a 50 mL round flask equipped with a septa and Nitrogen flow, dry ethylene glycol (0.31 g, 5.0 mmol) was added. Then, dry toluene (15 mL) was added following by triisopropyl borate (1.17 mL, 5.1 mmol). The reaction mixture was gently heated to reflux until a homogeneous colorless solution was formed. A solution of (S)-(-)-α,α -diphenyl-2-pyrrolidinemethanol (1.267 g, 5.0 mmol) in dry toluene (10 mL) was added to the reaction mixture while a white precipitate was observed during the addition. The resulting solution with the white solid was concentrated in the rotovaporator by heating at 80° C./20 mmHg for about 1 hour until all volatiles were evaporated. The white crystalline solid was dried overnight using high vacuum to remove toluene traces. A compound was obtained with quantitative yield (1.616 g).
The following example is about its application on the synthesis of proline-based N,N′-dioxide ligands for copper-catalyzed enantioselective Henry reactions. [6]
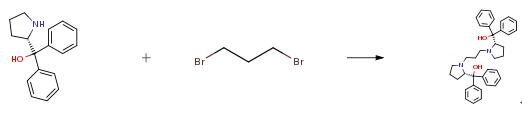
Amino alcohol (1.07 g, 8.3 mmol) was added to a 100 mL round-bottomed flask containing 50 mL acetonitrile, then 1,3-dibromopropane (0.83 g, 4.2 mmol) and potassium carbonate (3.50 g, 2.5 mmol) was slowly added to the flask over 10 min. The mixture was stirred overnight at 95°C, then cooled to room temperature. The solvent was removed in vacuo, water (20 mL) was added to the residue, and the mixture was extracted with ethyl acetate (3×10 mL). The combined organic phase was dried over Na2SO4, filtered and concentrated. The obtained amino alcohol was used without further purification. To a solution of the amino alcohol (0.95 g, 3.2 mmol) in 15 mL dichloromethane, was added m-chloroperoxybenzoic acid (1.61 g, 7.0 mmol). The mixture was stirred at room temperature for 12 h, and the solvent was removed in vacuo to give the crude product. The product was obtained in 80% (1.02g) after column chromatography through silica gel (ethyl acetate as eluent).
The following example is about its application on the synthesis of a heterogeneous and green hybrid catalyst for organic transformations [7]
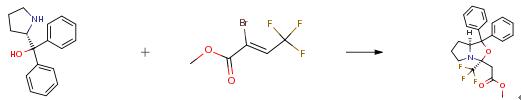
Under N2 atmosphere, Cs2CO3 (326 mg, 1.00 mmol) and L-prolinamide (0.6 mmol) in dry DMF (5 mL) were placed in a 10 mL Schlenk flask at rt. The suspension was stirred for 30 min. (Z)-methyl-2-bromo-4,4,4 -trifluorobut-2-enoate 1 (117 mg, 0.5 mmol) was added at this temperature. The solution was stirred until the reaction was completed (usually 3~5 h). The reaction was then quenched by adding saturated aqueous NH4Cl solution (10 mL). The mixture was extracted with EtOAc (3×10 mL). The combined organic layer was washed with brine and dried over Na2SO4. After the removal of solvents under vacuum, the crude product was further purified by silica gel column chromatography (PE:EA = 2:1 v/v) to afford pure products.
References
1. Stepanenko V, et al. Highly enantioselective carbonyl reduction with borane catalyzed by chiral spiroborate esters derived from chiral 1, 2-aminoalcohols[J]. Tetrahedron Asymmetry, 2006, 17(1):112-115.
2. Garrett CE, et al. The enantioselective reduction of 2'-fluoroacetophenone utilizing a simplified CBS-reduction procedure[J]. Tetrahedron Asymmetry, 2002, 13(13):1347-1349
3. Rostamnia S, Doustkhah E. Nanoporous silica-supported organocatalyst: a heterogeneous and green hybrid catalyst for organic transformations[J]. Royal Society of Chemistry Advances, 2014, 4(54):28238-28248.
4. Cui HF, Li YW, Zheng CW, Zhao G, Zhu SS, Enantioselective catalytic epoxidation of alpha, beta-enones promoted by fluorous alpha, alpha-diaryl-L-prolinols. Journal of Fluorine Chemistry, 2008, 129:45
5. Ortiz-Marciales M, Stepanenko V, Correa-Ramirez W, Jesus M de. Highly enantioselective carbonyl reduction with borane catalyzed by chiral spiroborate esters derived from chiral beta-aminoalcohols. US2008/200672[P], 2008, A1, Page column 3-4.
6. Gao E, Li M, Duan L, Li L, Li YM. Isosteric expansion of the structural diversity of chiral ligands: Design and application of proline-based N,N′-dioxide ligands for copper-catalyzed enantioselective Henry reactions[J]. Tetrahedron, 2019, 75(37):130492.
7. Zhang H, Shen Q, Lu L. Highly diastereoselective synthesis of optically pure trifluoromethyl- substituted imidazolidine, oxazolidine and thiazolidine. Tetrahedron Letters, 2011, 52(2):349-351.
You may like
Related articles And Qustion
See also
Lastest Price from (S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol manufacturers
![112068-01-6 diphenyl-[(2S)-pyrrolidin-2-yl]methanol](/ProductImageEN2/2025-10/Small/aedfcfa1-de66-4059-9e0b-deb9f6b56239.jpg)
US $0.00-0.00/kg2025-10-24
- CAS:
- 112068-01-6
- Min. Order:
- 100kg
- Purity:
- 99%min
- Supply Ability:
- 100 tons
US $0.00-0.00/mg2025-04-18
- CAS:
- 112068-01-6
- Min. Order:
- 10mg
- Purity:
- 95%+
- Supply Ability:
- 100000