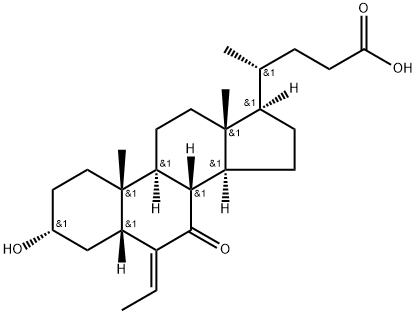
Preparation of (E)-3α-hydroxy-6-ethylidene-7-keto-5β-cholan-24-oic acid
(E)-3α-hydroxy-6-ethylidene-7-keto-5β-cholan-24-oic acid is an important organic intermediate to synthetize analogues of cholan-24-oic acid.
(E)-3α-hydroxy-6-ethylidene-7-keto-5β-cholan-24-oic acid can be prepared according to the reported literatures[1-3].
Method 1[1]

0.1 g (0.19 mmol) of 3α-(2,2-dimethylpropanoyl)-7α-hydroxy-(6E)-ethylidene-5β- cholan-24-oic acid methyl ester is dissolved in THF (1 ml) at room temperature and under nitrogen atmosphere in a 20 mL two-neck flask. 2-Iodoxybenzoic acid (IBX) adduct with quinoline (80 mg, 0.20 mmol) are added and the reaction mixture is heated to 50°C and stirred for 2 hour, then filtered and concentrated under reduced pressure. Then, the residue is diluted with DCM (2 ml) and washed with HC1 5percent (2 ml), NaHCO3 saturated solution (2 ml) and H2O (2 ml). The organic phase is concentrated under reduced pressure giving 105 mg of raw material that is dissolved in methanol (2 ml) and treated with NaOH 30 percent (0.5 ml). The solution is stirred for 48 hours, then acidified with HC1 37 percent until reaching pH 1 and concentrated under reduced pressure. The obtained residue is purified by silica gel chromatography (DCM:acetone = 95:5) giving 43 mg of 3a- hydroxy-7-oxo-(6E)- ethylidene-5P-cholan-24-oic acid with a yield of 53 percent. 1H-NMR (300 MHz, CDCl3) δ: 6.18 (1 H, q, J= 6.9 Hz), 3.70-3.58 (1H, m), 2.60-1.10 (29H, m), 1.00 (3H, s), 0.94 (3H, d, J=6.3 Hz), 0.64 (3H, s).
Method 2 [2-3]

Saponification of the starting material (258.37 g, 600 mmol, calculated as dried substance) was charged into an inert reactor. At a temperature of NMT 50 °C, residual amounts of solvent were distilled off under vacuum. The residue was dissolved in methanol (360 mL) and water (54 mL) and caustic soda 50percent wt. (54 mL) were added. The reaction mixture was heated up to 49 °C to 53 °C and stirred at this temperature for at least 2 hours. The pH of the reaction mixture is checked and verified to be > 12. The crude compound for crystallization can also be a mixture of E/Z isomers. Ethanol (390 to 520 mL) and the crude product (130 g) were charged into an inert reactor. To dissolve the crude compound, the reaction mixture was heated to reflux. Then, the reaction mixture was cooled in a controlled cooling ramp to 15 °C to 20 °C within 3 to 5 hours by a linear profile. The crystalline compound was isolated using a centrifuge and then washed with ethyl acetate (50-100 mL, 2 times). Drying was done in the tumble dryer under vacuum and at approximately 60 °C. This leads to 85.8 g (66percent) yield. A sample was taken to measure assay, purity, and moisture of the purified compound. The purified compound is the E isomer of 3a-hydroxy-6-ethylidene-7-keto-5p-cholan-24-oic acid. Isolation of the purified compound, the E isomer, can be optional. The E isomer and Z isomers have different solubilities. The E isomer is less soluable and crystallizes such that the Z isomer can be washed away.
To the above(E) -3α-hydroxy-6-ethylidene-7-one-5β-cholyl-24-methyl ester methanol (250 mL) was added to the crude crude product. Water (20 mL), sodium hydroxide (20 g, 0.5 mol), stirring reaction 3 h, recovery of methanol, adding dilute hydrochloric acid to adjust the pH to 3, precipitation crystallization, filtration, washing, drying, recrystallization from a mixture of ethyl acetate-acetonitrile (v / v = 1: 0.9)(E) -3α -hydroxy-6-ethylidene-7-one-5β-cholene-24-acid (32.1 g, Yield 77 percent to 3α-hydroxy-7-keto-5β-cholene-24-methyl ester).
References
1.Dipharma FSRL, Razzetti G, Iuliano A, Giannotti L, Iannucci G, Attolino E, Lucchini V, Graziosi R. Method for preparing a farnesoid x receptor agonist. WO2017/174515[P], 2017, A1, Page column 35.
2.Intercept Pharmaceuticals Inc. Steiner A, Waenerlund PH, Jolibois E, Rewolinski M, Gross R, Sharp E, Dubas-Fisher F, Eberlin A. Preparation, uses and solid forms of obeticholic acid. WO2013/192097[P], 2013, A1, Page column 60-62.
3.Suzhou Jingye Medicine & Chemical Co. Ltd. Shen J, Liu M. A (E)- 3 α - hydroxy - 6 - ethylidene - 7 - one - 5 β - cholane - 24 - acid preparation method (by machine translation). CN106083971[P], 2016, A, Paragraph 0023; 0024; 0026; 0027.
Lastest Price from (E)-3α-hydroxy-6-ethylidene-7-keto-5β-cholan-24-oic acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 1516887-33-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
![1516887-33-4 [C2OHMIM][DCA]](/ProductImageEN/2023-04/Small/92d60c6b-4c0a-47d1-8311-6d4e5db4e179.jpg)
US $0.00-0.00/KG2025-04-15
- CAS:
- 1516887-33-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg