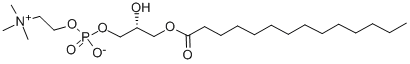
1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine- Reaction / Application on Synthetic Works
1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine is a chemically defined phosphatidylcholine, which may be used to compose the phospholipid bilayers of liposomes. In addition, it is also an important organic intermediate to synthetize substituted phosphatidylcholine.
The following example is about its application on the synthesis of unusual macrocyclic and bolaform phosphatidylcholines [1]
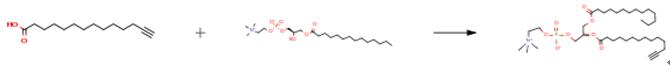
The acid (336 mg, 1.5 mmoles) and 1-myristoyl-2-hydroxy-sn-glycero-3- phosphocholine (0.5 mmol, 240 mg) were dried by azeotropic distillation with toluene (3×10 mL) under reduced pressure at rt, then at 30 °C overnight (1mmHg). The flask was then filled with argon, and 30 mL of dry CHC13 was injected. DCC (1.5 mmol, 309 mg) and DMAP (1.5 mmol; 183 mg) were added to the mixture. After 24 h, the mixture was acidified to pH 2 with aqueous 10% HCl, extracted with CHC13/MeOH (2:l) (3 × 150 mL), and washed with pH 2 MeOH/H20 (1:l). The organic phase was evaporated, dried by azeotropic distillation of toluene, and chromatographed using CHC13/MeOH (9:l) to elute the unreacted fatty acid and urea then CHCl3/MeOH/H2O( 70:26:4) to elute the phospholipid (308 mg; 91%). TLC (CHCl3/MeOH/H2O (70:26:4)), Rf :0.56.
The following example is about its application on the preparation of the polymerizable phospholipids [2]
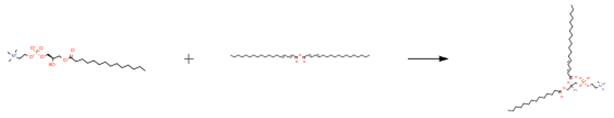
Into 2 L flasks was placed DMPC (45 g, 66.38 mmol) in 600 mL of Et2O:EtOH (95 : 5), 300 mL of 100 mM Tris-HCl buffer (pH 8.0), 100 mL of 100 mM CaCl2, and 15 mL of the phospholipase solution. The reaction mixture was suspended and stirred at room temperature for 19 h. The progress of the reaction was monitored by TLC (CHCl3:MeOH:H2O~65:25:4). Upon completion of the reaction, the solvent was evaporated under reduced pressure. The residual water phase was combined, and extracted three times with 500 mL of chloroform. To the water phase was added 500 mL of chloroform and 100 mL of methanol and the organic phase was separated; this procedure was repeated three times. Finally, the water phase was extracted three times with 500 mL of chloroform. The combined organic phase (ca. 4.5 L) was dried over 150 g of anhydrous Na2SO4 and evaporated. The residue was dried seven times by evaporation of 100 mL of dry benzene. The residue was purified by chromatography on 650 g silica gel eluted with CHCl3:MeOH gradient solvents (10:0, 9:1, 8:2, 7:3, 5: 5). The Rf: 0.07 (CHCl3:MeOH:H2O~65:25:4) fractions were collected and evaporated. The pure lysomyristoyl phosphatidylcholine gave a 78.6% yield (48.8 g, 0.1043 mol). The TLC (CHCl3:MeOH:H2O~65:25: 4) showed one spot (Rf :0.07).
In a 1 L flask was placed 66 wt% of (E,E)-octadeca-2,4-dienoic anhydride solution (ca. 0.1177 mol). Lysomyristoyl phosphatidylcholine (40.5 g, 86.66 mmol) and 8.44 g of DMAP (69.08 mmol) were added, and the gas in the reaction flask was replaced with nitrogen and the flask sealed. The reaction mixture was stirred for 2 days at 30 ³C. The progress of the reaction was monitored by TLC (CHCl3:MeOH:H2O~ 65:25:4). After the reaction, the solvent was evaporated in vacuo. The residue was divided into two portions, then each portion was chromatographed on 650 g silica gel with eluted CHCl3:MeOH gradient (10:0, 9:1, 8:2, 7:3, 5:5) using an open column (i.d. of 10 cm). The combined eluate of the Rf: 0.2 fractions of TLC was evaporated. The residue was purified on 100 g silica gel with eluted CHCl3:MeOH:H2O gradient (80:19:1, 80:18:2) by using medium-pressure chromatography (i.d. of 30 mm, length of 22 cm, eluent was CHCl3:MeOH:H2O~80:19:1, 80:18:2, pressure was 0.2 kgf cm-2, flow rate was 35 mL min-1, pump was Model-540 Yamazen). The eluate was poured through a column of ion-exchange resin to remove the DMAP. The resin was washed with 1 L of the MeOH and CHCl3 before use. The combined eluates were evaporated, and the residue was suspended in 200 mL of ultra-pure water. The suspension was frozen by liquid nitrogen, and the solid was freeze-dried in vacuo for 24 h. The pure product gave a 39.5% yield (25 g, 34.24 mmol): Rf~0.19 (CHCl3:MeOH:H2O~ 65:25:4)
References
1.Hebert B, Lennox J. A new reagent for the removal of the 4-methoxybenzyl ether: Application to the synthesis of unusual macrocyclic and bolaform phosphatidylcholines[J]. Journal of Organic Chemistry, 1992, 57(6):1777-1783.
2.Akama K, ano Y, Tokuyama S, Hosoi F, Omichi H. γ-Ray irradiation of liposomes of polymerizable phospholipids containing octadeca-2,4-dienoyl groups and characterization of the irradiated liposomes[J]. Journal of Materials Chemistry, 2000, 10(5):1047-1059
You may like
Lastest Price from 1-MYRISTOYL-SN-GLYCERO-3-PHOSPHOCHOLINE manufacturers
US $8.00-1.00/KG2024-03-29
- CAS:
- 20559-16-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $15.00-10.00/KG2021-07-13
- CAS:
- 20559-16-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton