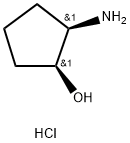
(1S,2R)-cis-2-Aminocyclopentanol hydrochloride- Reaction / Application on Synthetic Works
(1S,2R)-cis-2-Aminocyclopentanol hydrochloride is an important organic chiral building block, which is used for asymmetric synthesis.
The following example is about its application on the synthesis of pyrimidinyl indole compounds [1]
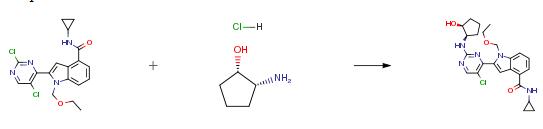
A mixture of N-cyclopropyl-2-(2,5-dichloropyrimidin-4-yl)-l-(ethoxymethyl)-lH- indole-4-carboxamide (10 g, 25 mmol), (1S,2R)-cis-2-Aminocyclopentanol hydrochloride (4.1 g, 30 mmol) and DIPEA (5 mL, 30 mmol) in DMSO (70 mL) is stirred at 100 °C for 3 h, then poured into water and extracted with EA. The combined extracts are washed with aqueous saturated sodium chloride, dried over Na2SO4 and concentrated in vacuo. The residue is purified by chromatography on silica gel to give 2-{5-chloro-2-[(25)-2- hydroxycyclopentylamino] pyrimidin-4-yl} -N-cyclopropyl- 1 -(ethoxymethyl)- lH-indole- 4-carboxamide (7 g, 60.3 %). The above product (7 g, 14.9 mmol) is stirred with hydrogen chloride (6 M in methanol, 210 mL, 1.26 mol) at 45 °C for 12 h. The precipitate is collected by filtration, washed with methanol and dried in vacuo to give the final product (4.7 g, 70 %).
The following example is about its application on the synthesis of 2-heteroaryl-3-oxo-2,3 -dihydropyridazine-4-carboxamides for the treatment of cancer [2]
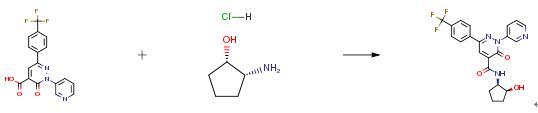
3-Oxo-2-(pyridin-3-yl)-6-[4-(trifluoromethyl )phenyl]-2 ,3-di hydropyridazine-4- carboxylic acid (50 mg, 0.138 mmol) was dissolved in anhydrous DMF (1.05 mL). (1S,2R)-2-Aminocyclopentanolhydrochloride (1:1) (38 mg, 0.28 mmol), N-ethyl-N-isopropylpropan-2-amine (157 pL, 0.90 mmol), and propane phosphonic acid anhydride (T3P, 122 pL, 50% in DMF, 208 pmol) were successively added. It was stirred for 2 h at rt. The reaction mixture was diluted with methanol and concentrated under vacuum. The residue was purified by RP-HPLC (column: X-Bridge 0185pm 100×30mm, mobile phase: (water + 0.2 vol% aqueous ammonia (32%)) acetonitrile, gradient) to afford 36.5 mg (59%) of the final product.
The following example is about its application on the synthesis of 3-oxo-2,6-diphenyl-2,3- dihydropyridazine-4-carboxamides [3]
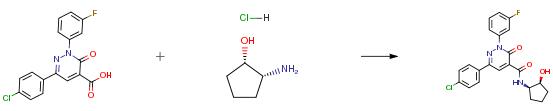
A solution of 75 mg the starting material, 59.9 mg (1S,2R)-2-amino-cyclopentanol hydrochloride (1 :1 ), 165.5 mg HATU, 0.1 1 mL ethyldiisopropylamine and 1 .3 mg 4-dimethylaminopyridine in 2 mL of DMF was stirred at room temperature for 3 hours. Then the reaction mixture was filtered and subjected to RP-HPLC (Instrument: Labomatic HD-3000 HPLC gradient pump, Labomatic Labocol Vario-2000 fraction collector; column: Chromatorex C-18 125 mm × 30 mm, eluent A: 0.1 % formic acid in water, eluent B: acetonitril; gradient: A 60% / B 40%→ A 20% / B 80%; flow: 150 mL/min; UV-detection: 254 nm) to yield 34 mg 6-(4-chlorophenyl)-2-(3- fluorophenyl)-N-[(1R,2S)-2-hydroxycyclopentyl]-3-oxo-2,3-dihydropyridazine-4-carboxamide.
The following example is about its application on the synthesis of sulfamoyl-arylamides as medicaments for the treatment of hepatitis b [4]
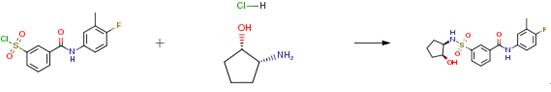
A tube was charged with 3-[(4-fluoro-3-methyl-phenyl)carbamoyl]- benzenesulfonyl chloride (250 mg, 0.76 mmol) and an amine (1.1 eq) and CH2C12 (5 mL) was added. The solution was stirred, DIPEA (329 μ, 1.9 mmol, 2.5 eq) was added and the mixture was further stirred for 30 minutes. Then, HC1 (1M aq / 5 mL) was added and the mixture was stirred for 5 minutes more. The formed precipitate was collected on a glass filter and rinsed with CH2CI2 (2 x 5 mL). The precipitate was further purified using silica gel column chromatography (gradient elution: EtOAc-heptane 0: 100 to 100:0). Drying in vacuo at 55°C resulted in the product as a bright white powder.
References
1.Hutchison Medipharma Limited. Su WG, Li J. Pyrimidinyl indole compounds.
WO2011/134140[P], 2011, A1, Page column 8.
2.Bayer Aktiengesellschaft, Bayer Pharma Aktiengesellschaft, Deutsches Krebsforschungszentrum. Gutcher I, Röhn U, Schmees N, Zorn L, Irlbacher H, Platten M. 2-heteroaryl-3-oxo-2,3-dihydropyridazine-4-carboxamides for the treatment of cancer. WO2018/146010[P], 2018, A1, Page column 221.
3.Bayer Pharma Aktiengesellschaft, Deutsches Krebsforschungszentrum (Dkfz). Schmees N, Gutcher I, Irlbacher H, Bader B, Zhao N, Platten M. 3-oxo-2,6-diphenyl-2,3-dihydropyridazine-4-carboxamides. WO2017/202816[P], 2017, A1, Page column 182.
4.Janssen R&D Ireland, Last SJ, Raboisson PJMB, Rombouts G, Vandyck K, Verschueren WG. Sulfamoyl-arylamides and the use thereof as medicaments for the treatment of hepatitis b. WO2014/33170[P], 2014, A1, Page column 78, 83.
You may like
Lastest Price from cis-(1S,2R)-2-Aminocyclopentanol hydrochloride manufacturers

US $0.00-0.00/KG2021-04-08
- CAS:
- 225791-13-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 200kg/month