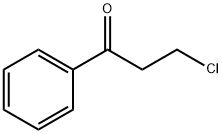
3-Chloropropiophenone: synthesis and application
Introduction
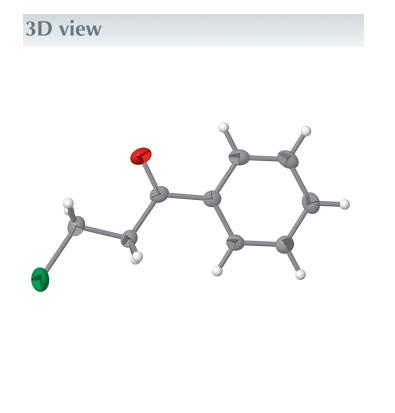
The title compound, 3-chloropropiophenone (or 3-chloro-1-phenylpropan-1-one), C9H9ClO, consists of an almost planar molecule that is charaterized by very small torsion angles within the alkyl side chain (torsion angles < 6.3°). No hydrogen bonds are observed in the crystal packing. The compound exhibits amelting point of 54℃. The molecular structure of 3-chloropropiophenone is almost planar with torsion angles of less than 6.3 degrees [maximum torsion angle: C1—C2—C3—O1 =- 6.21 (19)°] in the side chain (Fig. 1)[1]
Synthesis and crystallization
3-Chloropropiophenone was obtained as colourless crystals in quantitative yield from the Friedel–Crafts acylation of benzene and 3-chloropropionyl chloride in dichloromethane. AlCl3 (38.2 g, 286.5mmol, 1.25 eq.) was suspended in 50 ml of dry dichloromethane at 0°C. A solution of 3-chloropropionyl chloride (29.1 g, 229.2 mmol, 1.0 eq.) in 90ml dichloromethane was added dropwise at 0℃ to the AlCl3 suspension. Afterwards, a solution of benzene (17.9 g,229.2 mmol, 1.0 eq.) in 25 mL dichloromethane was addeddropwise at 0℃ to the suspension and further stirred for 2 h at 0℃ and 12 h at ambient conditions. The final solution was poured onto ice and concentrated hydrochloric acid (70 g:7 g) and after separation of the organic phase, the aqueous phasewas extracted twice with 100 ml portions of dichloromethane.The combined organic phases were extracted twice with 150 ml portions of water and finally dried over Na2SO4. Thesolvent was removed completely under diminished pressureand the off-white crystalline solid residue was recrystallizedfrom pentane to yield the final product (37.5 g, 97%).Colourless single crystals of 3-chloropropiophenone wereobtained from a pentane solution by slow evaporation of thesolvent at 4℃ over the period of one week. Analytic data for C9H9ClO: m.p. 54℃, elemental analysis % (calculated):C 64.14 (64.11), H 5.25 (5.38); Cl 21.01 (21.02). 1H-NMR(400 MHz, CDCl3): δ (p.p.m.) = 7.98-7.93 (m, 2H, ArH); 7.61-7.56 (m, 1H, ArH); 7.51-7.45 (m, 2H, ArH); 3.92 (t,3J = 6.8 Hz,2H); 3.45 (t, 3J = 6.7 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ(p.p.m.) = 196.78 (CO); 136.45 (C); 133.65, 128.84, 128.84,128.14, 128.14 (CH); 41.36, 38.79 (CH2).[1]
Enhanced whole-cell biotransformation
This study focuses on dehalogenation of halogenated organic substrate (3-Chloropropiophenone) using both free and hydrogel entrapped microalgae Chlorella emersonii (211.8b) as biocatalyst. We aimed at successful immobilization of C. emersonii (211.8b) cells and to assess their biotransformation efficiency. Results: Aquasorb (entrapping material in this study) was found to be highly biocompatible with the cellular growth and viability of C. emersonii. A promising number of entrapped cells was achieved in terms of colony-forming units (CFUs = 2.1×104) per hydrogel bead with a comparable growth pattern to that of free cells. It was determined that there is no activity of hydrogenase that could transform 1-phenyl-2-propenone into 1-phenyl-1-propanone because after 12 h the ratio between two products (0.36 ± 0.02) remained constant throughout. Furthermore, it was found that the entrapped cells have higher biotransformation of 3-chloropropiophenone to 1-phenyl-1-propanone as compared to free cells at every interval of time. 1-phenyl-2-propenone was excluded from the whole-cell biotransformation as it was also found in the control group (due to spontaneous generation). Conclusion: Hence, enhanced synthesis of 1-phenyl-1-propanone by entrapped Chlorella (211.8b) can be ascribed to either an enzymatic activity (dehalogenase) or thanks to the antioxidants from 211-8b, especially when they are in immobilized form. The aquasorb based immobilization of microalgae is highly recommended as an effective tool for exploiting microalgal potentials of biocatalysis specifically when free cells activities are seized due to stress.[2]
Asymmetric reduction of (S)-3-chloro-1-phenylpropanol
A number of experiments were conducted to optimize the biocatalyst and reaction conditions for the asymmetric reduction of 3-chloropropiophenone.The effects of resting cells and the immobilized cells,the preincubation time, thermal pretreatment time, the size of the alginate beads, and the multiplication culture time were studied. The standard conditions for these experiments are summarized as follows:biotransformation time, 48 h; substrate concentration,1gl-1; pH, 8.0; temperature, 35℃; shaking at 120 rpm; sodium alginate concentration, 2.5%. An efficient method for asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone was developed using preheated Candida utilis cells immobilized in calcium alginate gel beads. Heating the immobilized cells (bead diameter 1.5 mm) at 45℃ for 50 min allowed the reaction to proceed with 99.5% enantiomeric excess (ee) and an 85% yield with 1 g substrate l-1 (batch addition in three aliquots) in 48 h. The immobilized cells retained approximately 50% of their original catalytic activity after being reused three times.[3]
3-Chloropropiophenone derivatives application
No clinically approved therapies are currently available that prevent the onset of photoreceptor death in retinal degeneration. Signaling between retinal neurons is regulated by the release and uptake of neurotransmitters, wherein GABA is the main inhibitory neurotransmitter. In this work, novel 3-chloropropiophenone derivatives and the clinical anticonvulsants tiagabine and vigabatrin were tested to modulate GABA signaling and protect against light-induced retinal degeneration. Abca4-/-Rdh8-/- mice, an accelerated model of retinal degeneration, were exposed to intense light after prophylactic injections of one of these compounds. Imaging and functional assessments of the retina indicated that these compounds successfully protected photoreceptor cells from degeneration to maintain a full-visual-field response. Furthermore, these compounds demonstrated a strong safety profile in wild-type mice and did not compromise visual function or damage the retina, despite repeated administration. These results indicate that modulating inhibitory GABA signaling can offer prophylactic protection against light-induced retinal degeneration.-Schur, R. M., Gao, S., Yu, G., Chen, Y., Maeda, A., Palczewski, K., Lu, Z.-R. New GABA modulators protect photoreceptor cells from light-induced degeneration in mouse models.[4]
References
1.Sonneck M, Spannenberg A, Wohlrab S, Peppel T. 3-Chloro-propio-phenone. IUCrdata. 2025;10(Pt 4):x250349. Published 2025 Apr 29. doi:10.1107/S2414314625003499
2.Khan S, Fu P, Di Fonzo A, Marasca I, Secundo F. Enhanced whole-cell biotransformation of 3-chloropropiophenone into 1-phenyl-1-propanone by hydrogel entrapped Chlorella emersonii (211.8b). Biotechnol Lett. 2021;43(12):2259-2272. doi:10.1007/s10529-021-03194-y
3.Gen-Sheng Y, Jiang-Yan X, Zhi-Min O, Shan-Jing Y. Asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone by preheated immobilized Candida utilis. Biotechnol Lett. 2009;31(12):1879-1883. doi:10.1007/s10529-009-0084-4
4.Schur RM, Gao S, Yu G, et al. New GABA modulators protect photoreceptor cells from light-induced degeneration in mouse models. FASEB J. 2018;32(6):3289-3300. doi:10.1096/fj.201701250RSee also
Lastest Price from 3-Chloropropiophenone manufacturers

US $10.00/ASSAYS2025-08-29
- CAS:
- 936-59-4
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00-0.00/KG2025-04-21
- CAS:
- 936-59-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt