Application of 1,1-Cyclobutanedicarboxylic acid in organic synthesis
Introduction
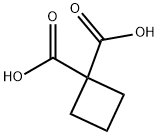
1,1-Cyclobutanedicarboxylic acid (also referred to as cyclobutane-1,1-dicarboxylic acid;Figure 1) serves as an intermediate in the synthesis of anticancer drugs, such as carboplatin and cisplatin. This compound remains stable under normal temperature and pressure conditions, crystallizing into a prismatic form. When heated to temperatures ranging from 210 to 220°C, it decomposes to form cyclobutanecarboxylic acid. 1,1-Cyclobutanedicarboxylic acid is soluble in water, ether, chloroform, and benzene, and exhibits corrosive properties. 1,1-Cyclobutanedicarboxylic acid finds extensive applications in industry, particularly in the realm of metal carboxylates. Owing to its diverse biological characteristics, it plays a crucial role as a significant ligand.[1]

Diamides of 1,1-Cyclobutanedicarboxylic acid
Zirvi et al. have reported several diimides of 1,1-Cyclobutanedicarboxylic acid to be inactive either as general CNS depressants or potentiators of barbiturate sedation. Of the compounds studied only cyclobutane-1,5-spiro-2,6-diketo-4-thiohexahydropyrimidine was aective, Its effect was that of barbiturate potentiation. This compound has prompted consideration of various other 1,1-cyclobutanedicarboxylic acid derivatives.[2] In addition,a group of nine diamides of 1,1-Cyclobutanedicarboxylic acid has been prepared and examined for general depressant and analgesic activity. Several showed activity in both respects but with little overlap. Several compounds were evaluated for anticonvulsant and myorelaxant activity. No activity was found.[3]
Neutral complexes Synthesis
Two neutral complexes of formula [M(bpy)(cbdca)] [where M is palladium(II) (Pd(II)) or platinum(II) (Pt(II)), bpy is 2,2'-bipyridine and cbdca is anion of 1,1-cyclobutanedicarboxylic acid] have been synthesized. These water soluble complexes have been characterized by chemical analysis and conductivity measurements as well as 1H-NMR, ultraviolet-visible and infrared spectroscopy. In these complexes the ligand cbdca coordinates to Pt(II) or Pd(II) as bidentate with two oxygen atoms. They are nonelectrolyte in conductivity water. These complexes inhibit the growth of P388 lymphocytic leukemia cells and their targets are DNA. They invariably show ID50 values less than cisplatin. [Pt(bpy)(cbdca)] and [Pd(bpy)(cbdca)] have been interacted with calf thymus DNA and bind to DNA through coordinate covalent bond. In addition, the influence of binding of these complexes on the intensity of EtBr-DNA have been studied. They bind to DNA via a nonintercalating mode.[4]
Platinum(II) complexes with malonic acids synthesis
Four Pt(II) complexes of the general formula [Pt(L)(5,6-epoxy-1,10-phen)], where L is an anion of either malonic acid (mal, Pt1), 2-methylmalonic acid (Me-mal, Pt2), 2,2-dimethylmalonic acid (Me 2-mal, Pt3) or 1,1-cyclobutanedicarboxylic acid (CBDCA, Pt4) and 5,6-epoxy-1,10-phen is 5,6-epoxy-5,6-dihydro-1,10-phenanthroline, were synthesized and characterized by elemental microanalysis and different spectroscopic techniques. The crystal structure of anhydrous Pt3 complex was determined by single crystal X-ray diffraction. The in vitro anticancer activity of the platinum(II) complexes was investigated in human and murine cancer cell lines as well as in a normal murine cell line by MTT assay. The results show that the investigated platinum(II) complexes exhibit potent cytotoxic activity against murine breast carcinoma cells (4T1), human (HCT116) and murine (CT26) colorectal carcinoma cells. The Pt3 complex shows stronger selectivity against cancer cells compared to other platinum(II) complexes tested and thus exhibits beneficial antitumor activity, mainly by inducing apoptosis and inhibiting cell proliferation and migration. The Pt3 complex also exhibits significant in vivo antitumor activity in the orthotopical 4T1 tumor model without detected liver, kidney, lung, and heart toxicity. All the results indicate that these novel platinum(II) complexes have good antitumor activity on breast and colorectal cancer and have the potential to become possible candidates for cancer treatment.[5]
Characterization of (2-Picolylamine)(1,1-cyclobutanedicarboxylato) palladium(II)
The influence of a 2-picolylamine (pic) ligand on the reactivity of Pd(II) complexes was investigated by detailed equilibrium and kinetic studies. Reactions of [Pd(pic)(H2O)2]2+ with chloride, 1,1-cyclobutanedicarboxylic acid (CBDCAH2), inosine (ino), and inosine 5'-monophosphate (5'-IMP) were studied. A significantly higher reactivity for the first reaction step involving the displacement of one coordinated solvent molecule on [Pd(pic)(H2O)2]2+ was observed for the nucleoside inosine (k10℃= 25 400±200 M-1 s-1) than for the nucleotide 5'-IMP (k10℃=7100±300M-1 s-1) and for CBDCAH(-) (k25℃= 5380±70 M-1 s-1); DeltaH=54±2 kJ mol-1; DeltaS=10± 4 J K-1mol-1; DeltaV=-0.2±0.7 cm3mol-1). The results are compared and discussed in reference to data reported for closely related systems in the literature. The molecular structure of [Pd(pic)(CBDCA)] in solution and in the solid state was resolved. [Pd(pic)(CBDCA)].2H2O crystallizes in the space group P2(1)/c (monoclinic, a = 5.659(5) Å, b = 18.320(5) Å, c = 14.027(5) Å, beta = 97.748(5) degrees, V = 1440.94(14) Å(3), Z=4).[6]
References
[1]. Zhang J. Preliminary Design of the Experimental Scheme of"The Preparation of 1,1- - Cyclic Butyl Two Formic Acid"[J].The Guide of Science & Education,2016,(15):35-36.DOI:10.16400/j.cnki.kjdkx.2016.05.018.
[2]. Zirvi KA, Jarboe CH. Diamides of cyclobutane-1,1-dicarboxylic acid. J Med Chem. 1969;12(5):926-927. doi:10.1021/jm00305a056
[3]. Zirvi KA, Jehangir S, Jarboe CH. Diamides of cyclobutane-1,1-dicarbocylic acid. II. Farmaco Sci. 1976;31(7):546-548.
[4]. Mansuri-Torshizi H, Ghadimy S, Akbarzadeh N. Synthesis, characterization, DNA binding and cytotoxic studies of platinum(II) and palladium(II) complexes of the 2,2'-bipyridine and an anion of 1,1-cyclobutanedicarboxylic acid. Chem Pharm Bull (Tokyo). 2001;49(12):1517-1520. doi:10.1248/cpb.49.1517
[5]. Dimitrijevi? Stojanovi? MN, Franich AA, Juri?evi? MM, et al. Platinum(II) complexes with malonic acids: Synthesis, characterization, in vitro and in vivo antitumor activity and interactions with biomolecules. J Inorg Biochem. 2022;231:111773. doi:10.1016/j.jinorgbio.2022.111773
[6].Rau T, Shoukry M, van Eldik R. Complex Formation and Ligand Substitution Reactions of (2-Picolylamine)palladium(II) with Various Biologically Relevant Ligands. Characterization of (2-Picolylamine)(1,1-cyclobutanedicarboxylato)palladium(II). Inorg Chem. 1997;36(7):1454-1463. doi:10.1021/ic961192v
You may like
Related articles And Qustion
Lastest Price from 1,1-Cyclobutanedicarboxylic acid manufacturers

US $0.00/MG2025-04-21
- CAS:
- 5445-51-2
- Min. Order:
- 100MG
- Purity:
- 98.0%
- Supply Ability:
- 1kg/month
US $100.00/KG2025-04-21
- CAS:
- 5445-51-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON