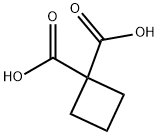
The synthesis method of 1,1-Cyclobutanedicarboxylic acid
1,1-Cyclobutanedicarboxylic acid was prepared by hydrolysis of the ethyl ester or half nitrile, 1-cyano-1-carboxycyclobutane. The ethyl ester has been prepared by condensation of ethyl malonate with trimethylene bromide, or chlorobromide, and by the action of sodium ethoxide on diethyl γ-bromopropylmalonate. The half nitrile was prepared by condensing trimethylene bromide with ethyl cyanoacetate, followed by hydrolysis of the ester to the acid.

In a 3-l three-necked round-bottomed flask, carrying a separatory funnel, a mechanical stirrer, a reflux condenser fitted with a calcium chloride tube, and a thermometer, are placed 160 g (1 mol) of ethyl malonate and 212 g (1.05 mol) of trimethylene bromide. The stirrer is started, and a solution of 46 g (2 gram atoms) of sodium in 800 ml. of absolute ethanol is added through the separatory funnel while the temperature of the reaction mixture is kept at 60–65℃. After this point, the remainder of the ethoxide solution is added rapidly enough to keep the reaction mixture at 60–65℃; this part of the addition requires about 30 minutes.
The reaction mixture is allowed to stand until the temperature drops to 50–55℃, after which it is heated in a steam bath until a sample added to water is neutral to phenolphthalein (about 2 hours). Water is added to dissolve the precipitate of sodium bromide, and the ethanol is removed by distillation. The flask is now arranged for steam distillation, and the ethyl 1,1-cyclobutanedicarboxylate and unchanged malonic ester are removed by steam distillation; about 4 l of distillate is collected. The ester layer in the distillate is separated, and the aqueous layer is extracted once with 1 l of ether. The extract and the ester layer are combined, and the ether is removed from the steam bath.
The esters are hydrolyzed by refluxing them for 2 hours with a 112 g of potassium hydroxide solution in 200 ml of ethanol. Most of the ethanol is removed by distillation, and the mixture is then evaporated to dryness in a steam bath. The residue is dissolved in the minimum amount of hot water (100 to 125 ml), and concentrated hydrochloric acid (about 90–95 ml) is added until the solution is slightly acidic. After the solution has been boiled for a few minutes to remove carbon dioxide, it is made slightly alkaline with ammonia. A slight excess of barium chloride is added to the boiling solution. The hot solution is filtered to remove barium malonate, the filtrate is cooled, and 100 ml of 12 N hydrochloric acid is added. The solution is then extracted with four 250-ml portions of ether. The extracts are combined and dried over calcium chloride, and the ether is removed by distillation in a steam bath. The residual pasty mass (about 38 g) is pressed on a porous plate to remove adherent oil and then dissolved in 30–50 ml of hot ethyl acetate. When cooled in an ice-salt bath, the solution deposits the pure dicarboxylic acid. This is filtered; the filtrate, when evaporated, yields a pasty mass of acid, which, in turn, is crystallized from ethyl acetate. The yield of pure 1,1-cyclobutanedicarboxylic acid melting at 156–158℃ is 30–34 g.
References
[1] Organic Syntheses Procedure (orgsyn.org) https://www.orgsyn.org/demo.aspx?prep=CV3P0213#:~:text=1%2C1-Cyclobutanedicarboxylic%20acid%20has,-cyano-1-carboxycyclobutane.
You may like
Lastest Price from 1,1-Cyclobutanedicarboxylic acid manufacturers

US $0.00/MG2025-04-21
- CAS:
- 5445-51-2
- Min. Order:
- 100MG
- Purity:
- 98.0%
- Supply Ability:
- 1kg/month
US $100.00/KG2025-04-21
- CAS:
- 5445-51-2
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON