Dodecylbenzenesulfonic Acid: Synthesis, Composition, Applications, and Storage
Introduction
Dodecylbenzene sulfonic acid (DBSA), a compound of significant industrial relevance, stands as a cornerstone in the production of detergents and various chemical formulations. Renowned for its superior surfactant properties, DBSA plays a crucial role in enhancing the cleaning efficacy of products it is incorporated into. Its chemical stability and compatibility with other detergent components make it an ideal choice for a wide range of applications, from household cleaning agents to industrial degreasers. As environmental and safety regulations within the chemical industry evolve, DBSA's adaptability allows it to meet stringent requirements, making it a key player in sustainable chemical solutions[1].

Figure 1 Characteristics of Dodecylbenzene Sulfonic Acid
Synthesis
The synthesis of dodecylbenzenesulfonic acid primarily involves the sulfonation of dodecylbenzene with sulfuric acid or oleum. This process, performed under controlled temperatures, yields DBSA as a complex mixture of linear alkyl benzene sulfonates. The reaction specifics, such as temperature, reactant ratio, and time, are crucial for determining the quality and yield of the final product. Advanced techniques in synthesis aim to optimize these parameters to enhance efficiency and reduce by-product formation.
Main Components
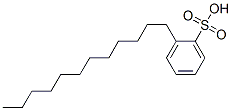
DBSA is characterized by its long-chain alkyl group attached to a benzene ring, which is further linked to a sulfonic acid group. This structure is pivotal for its surfactant properties, allowing it to reduce surface tension effectively, thus enhancing the mixing of water with oils and fats. The alkyl chain length and the degree of branching can vary, affecting the solubility and surface activity of the sulfonic acid. These variations allow for customization of DBSA to suit specific industrial needs, optimizing performance in different environments. The purity of DBSA is also a critical factor, influencing its effectiveness in various applications. Impurities can impact the consistency and safety of the final product, thereby dictating stringent control measures during manufacturing.
Applications
Dodecylbenzene sulfonic acid serves a vital role across several sectors, showcasing its versatility and essential functionality. Predominantly, it is used in the manufacture of household and industrial detergents due to its excellent surfactant properties, which enhance the cleaning power by effectively reducing the surface tension of liquids. DBSA is also employed in the synthesis of emulsifiers, wetting agents, and dispersants, crucial for products that require the stable mixing of otherwise immiscible substances. In the realm of personal care, DBSA is found in formulations for shampoos and soaps where mildness and effective cleansing are desired.
Beyond its primary use in cleaning agents, DBSA finds diverse applications in the oil recovery industry as a component in drilling fluids. Here, its properties help reduce the friction in drilling operations, facilitating smoother processes and protecting machinery. Additionally, in the agricultural sector, DBSA serves as a soil conditioner, improving the soil structure and increasing nutrient availability to plants. This not only enhances crop yield but also contributes to sustainable farming practices. Its broad spectrum of applications underscores its integral role in both everyday products and specialized industrial solutions.
A chemical oxidative polymerization of aniline dodecyl benzenesulfonic acid ANIDBSA and aniline hydrochloric acid ANIHCl was performed in an aqueous solution because the former monomer stimulates the solubility and the latter the conductive structure of the Ž .synthesized polymer. A co-doped polyaniline PANI was thus obtained, which is soluble in common organic solvents such as chloroform, and exhibits, without any additional doping, a higher conductivity than the insoluble HCl-doped PANI compressed pellet, and much higher conductivity than that prepared from pure ANIDBSA. The PANI doped with DBSA and HCl was characterized using FTIR, EDX and UV spectroscopies. At an ANIHClrANIDBSA molar ratio in the feed of 3:7 and no additional HCl, the polymer exhibited a maximum conductivity of 7.9 Srcm and a maximum yield of 30.8%. If additional HCl was introduced, the conductivity could reach a value as high as 14 Srcm.
The esterification of oleic acid with methanol by homogeneous acid catalysis was investigated, using 4-dodecylbenzenesulfonic acid as catalyst. First, the catalytic activity of 4-dodecylbenzenesulfonic acid in this reaction was compared with that of p-toluenesulfonic and sulfuric acid, and it was found that the reaction rate clearly increased with increasing hydrophobicity of the catalyst. Second, the effects of the catalyst/acid molar ratio, the methanol/oleic acid molar ratio, the water content, temperature, the stirring speed and the presence of triglycerides on the kinetics and the equilibrium of this esterification were studied. The main observations were that temperature and the proportion of catalyst had a positive effect on the kinetics, i.e., an increase in any of these factors led to an increase in the reaction rate. Methanol had an almost negligible effect. By contrast, water had a negative effect due to the formation of an aqueous phase, which removed part of the catalyst, thus reducing its concentration in the organic phase and hence the reaction rate. Third, the kinetics of esterification was studied in a wide range of operating conditions and a reversible second-order model was obtained that included the effect of the separation of the aqueous phase from the reaction mixture and hence the progressive decrease in the concentration of catalyst, methanol and the volume of the reaction mixture. This kinetic model adequately predicts the experimental data within the range of operation conditions investigated. The activation energy for the forward reaction was 58.5 kJ/mol, and 63.4 kJ/mol for the reverse reaction.
Storage
The storage of dodecylbenzene sulfonic acid requires careful handling to maintain its chemical integrity and ensure safety. DBSA should be stored in a cool, dry, and well-ventilated area away from incompatible substances such as bases and oxidizing agents, which can cause dangerous reactions. Appropriate materials for containers include stainless steel and certain types of plastics that resist corrosion. To prevent accidents, safety measures should also include spill management strategies and the use of proper personal protective equipment (PPE) for handling the acid, ensuring that those who interact with DBSA are adequately protected.
References
[1]Yin, Wusheng, and Eli Ruckenstein. "Soluble polyaniline co-doped with dodecyl benzene sulfonic acid and hydrochloric acid."Solution and Surface Polymerization. CRC Press, 2019. 275-286.
[2]Alegría, Alexandra, and Jorge Cuellar. "Esterification of oleic acid for biodiesel production catalyzed by 4-dodecylbenzenesulfonic acid."Applied Catalysis B: Environmental179 (2015): 530-541.
You may like
Lastest Price from Dodecylbenzenesulphonic acid manufacturers

US $0.00-0.00/KG2025-07-23
- CAS:
- 27176-87-0
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/kg2025-04-21
- CAS:
- 27176-87-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt