Itaconic Acid: Antimicrobial Activities and Biosynthetic Pathways
General Description
Itaconic acid is a potent antimicrobial compound, primarily inhibiting the glyoxylate shunt in bacteria like Mycobacterium tuberculosis and Salmonella enterica, thereby disrupting their ability to utilize alternative carbon sources when glucose is scarce. It functions as a competitive inhibitor of key metabolic enzymes such as isocitrate lyase and methylisocitrate lyase. Moreover, itaconic acid also affects the citramalate cycle by blocking propionyl-CoA carboxylase activity. While it is produced by fungi like Aspergillus itaconicus and mammalian immune cells, some pathogens have evolved detoxification mechanisms to counter itaconic acid's effects, highlighting an ongoing evolutionary struggle between host defenses and bacterial adaptations.

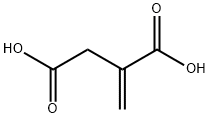
Figure 1. Itaconic acid
Antimicrobial Activities
Role in Inhibiting the Glyoxylate Shunt
Itaconic acid plays an important role as a bioactive compound in living organisms: Itaconic acid inhibits the glyoxylate shunt essential for many bacteria to survive during infection. The activation of the glyoxylate shunt is a survival strategy of invading pathogens, allowing them to utilize acetyl-CoA-generating carbon sources such as fatty acids or cholesterol for biomass production under glucose-limiting conditions. As an example, this metabolic mechanism is required for persistence of the pathogens Salmonella enterica and Mycobacterium tuberculosis within macrophages, but it is not essential for acute-phase infections.
The glyoxylate shunt is an anabolic variation of the TCA cycle. In contrast to the net decarboxylation of acetyl-CoA in the TCA cycle, the glyoxylate shunt preserves the carbon atoms of acetyl-CoA by bypassing the two decarboxylation steps. Isocitric acid is converted to glyoxylic acid and succinic acid, a reaction catalyzed by isocitrate lyase (ICL). Malate synthase further converts glyoxylic acid and another acetyl-CoA molecule to malic acid.
Itaconic acid inhibits ICL, the key enzyme of the glyoxylate shunt, and, in turn, the glyoxylate shunt itself. It functions as a competitive inhibitor, competing with succinic acid, presumably by binding to the active site of the enzyme. Although ICL and the glyoxylate shunt are present in many bacteria, archaea, plants, fungi, and protists, there is controversy over whether they are present in vertebrates. Because of its exclusive presence in microbes, this shunt has an important role as a potential drug target.1
Antimicrobial Properties Against Specific Pathogens
Studies have demonstrated that itaconic acid can inhibit bacterial growth effectively. For instance, research conducted in 2011 indicated that elevated levels of itaconic acid are produced by mammalian immune cells following infection, particularly in the lungs during M. tuberculosis infection. Itaconic acid concentrations of 25 to 50 millimoles per liter resulted in complete inhibition of M. tuberculosis growth in laboratory settings, while lower concentrations significantly impaired the growth of Salmonella enterica. The primary mechanism involves the inhibition of key metabolic enzymes, including isocitrate lyase and methylisocitrate lyase. By disrupting these pathways, itaconic acid prevents pathogen adaptation to alternative carbon sources, making it a potent agent in immune defense against invading pathogens. 1
Dual Role and Pathogen Adaptation Mechanisms
Interestingly, itaconic acid not only inhibits the glyoxylate shunt but also affects the citramalate cycle by blocking propionyl-CoA carboxylase activity. This broad-spectrum inhibitory action impairs various metabolic pathways in bacteria that lack isocitrate lyase activity, suggesting a broad potential for itaconic acid as an antimicrobial agent. However, some pathogens, such as Yersinia pestis and Pseudomonas aeruginosa, have developed mechanisms to detoxify itaconic acid through the action of specific enzymes, thereby facilitating their survival within host organisms. The identification of these detoxification genes underscores the evolutionary arms race between host defenses and pathogen adaptations. Despite the challenges posed by these adapted bacteria, itaconic acid remains a promising subject of study in the search for novel antimicrobial compounds, especially given its unique modes of action that target critical metabolic pathways in bacterial pathogens. 1
Biosynthetic Pathways
Discovery and Natural Producers
Itaconic acid, an important compound with various industrial applications, was first identified in 1836 by Baup during the distillation of citric acid. In 1840, Crasso synthesized it from cis-aconitic acid through decarboxylation, ultimately giving it the name itaconic acid, derived as an anagram of cis-aconitic acid. The significant biological discovery related to itaconic acid came in 1931 when it was identified as a metabolite produced in vivo by the fungus Aspergillus itaconicus. This discovery paved the way for recognizing several other natural producers of itaconic acid, including Aspergillus terreus and yeasts such as Candida sp. Notable advancements occurred when researchers, starting in 2011, demonstrated that mammalian immune cells also possess the ability to synthesize itaconic acid, highlighting its broader biological relevance. 2
Biosynthetic Pathways in Aspergillus terreus
The biosynthesis of itaconic acid in Aspergillus terreus involves several metabolic pathways, with considerable interest in understanding its biochemical precursors. Initial hypotheses suggested itaconic acid could originate from cis-aconitic acid, citramalic acid, or 1,2,3-tricarboxypropane. Further research, particularly by Bentley and Thiessen in 1957, established that is likely formed from cis-aconitic acid via decarboxylation catalyzed by the enzyme cis-aconitic acid decarboxylase, encoded by the gene cadA. This process begins with glucose metabolism, leading to the production of pyruvic acid and acetyl-CoA, with aconitase facilitating the conversion of citric acid into the precursor, cis-aconitic acid. The catalytic activity of aconitase and another enzyme, citrate synthase, is crucial for itaconic acid production, as they orchestrate the transformation of substrates into itaconic acid. Factors governing this pathway remain an area of active research, particularly in how oxaloacetic acid influences the regulation of itaconic acid synthesis compared to citric acid production in related fungi. 2
References:
[1] THEKLA CORDES K H Alessandro Michelucci. Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian Antimicrobial Metabolite.[J]. Annual review of nutrition, 2015, 35. DOI:10.1146/annurev-nutr-071714-034243.[2] MEILIN ZHAO. Itaconic acid production in microorganisms.[J]. Biotechnology Letters, 2018, 40 3. DOI:10.1007/s10529-017-2500-5.
You may like
Lastest Price from Itaconic acid manufacturers

US $1.00/PCS2025-04-21
- CAS:
- 97-65-4
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 97-65-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month