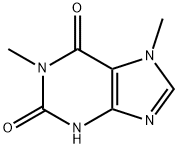
The Synthesis and Hazards of 1,7-Dimethylxanthine
1,7-Dimethylxanthine is a metabolite of caffeine and theobromine in animals. It has a role as a central nervous system stimulant, a human xenobiotic metabolite, a human blood serum metabolite and a mouse metabolite.

Synthesis
1,7-Dimethylxanthine is not known to be produced by plants but is observed in nature as a metabolite of caffeine in animals and some species of bacteria.
Metabolism
1,7-Dimethylxanthine is the primary metabolite of caffeine in humans and other animals, such as mice.Shortly after ingestion, roughly 84% of caffeine is metabolized into 1,7-Dimethylxanthine by hepatic cytochrome P450, which removes a methyl group from the N3 position of caffeine. After formation, 1,7-Dimethylxanthine can be broken down to 7-methylxanthine by demethylation of the N1 position, which is subsequently demethylated into xanthine or oxidized by CYP2A6 and CYP1A2 into 1,7-dimethyluric acid. In another pathway, 1,7-Dimethylxanthine is broken down into 5-acetylamino-6-formylamino-3-methyluracil through N-acetyl-transferase 2, which is then broken down into 5-acetylamino-6-amino-3-methyluracil by non-enzymatic decomposition. In yet another pathway, 1,7-Dimethylxanthine is metabolized CYPIA2 forming 1-methyl-xanthine, which can then be metabolized by xanthine oxidase to form 1-methyl-uric acid.
Beneficial effect
Several epidemiological studies suggest a beneficial effect of coffee consumption on the formation and progression of fibrogenic diseases, particularly of the liver. Recent data now point to a modulation of transforming growth factor-β (TGF-β) signaling by 1,7-Dimethylxanthine(1,7-DMX), the demethylated primary metabolite of caffeine.
Hazards
There is some evidence that human exposure to the material may result in developmental toxicity. This evidence is based on animal studies where effects have been observed in the absence of marked maternal toxicity, or at around the same dose levels as other toxic effects but which are not secondary non-specific consequences of the other toxic effects.
Long term exposure to high dust concentrations may cause changes in lung function i.e. pneumoconiosis; caused by particles less than 0.5 micron penetrating and remaining in the lung.
Exposure to 1,7-Dimethylxanthine for prolonged periods may cause physical defects in the developing embryo (teratogenesis).
Related articles And Qustion
See also
Lastest Price from 1,7-Dimethylxanthine manufacturers

US $600.00-550.00/kg2025-04-21
- CAS:
- 611-59-6
- Min. Order:
- 1kg
- Purity:
- 98%+
- Supply Ability:
- EX:10

US $1500.00/KG2025-04-17
- CAS:
- 611-59-6
- Min. Order:
- 1KG
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min