6-Bromoquinoline: Synthesis, Detection Method and application
Introduction
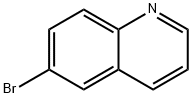
6-Bromoquinoline (Figure 1) is a structural fragment with important physiological activities, which is widely used in the synthesis of pharmaceutical intermediates, such as anticancer drug enhancers, receptor tyrosine kinase inhibitors and cardiotonics. 6-Bromoquinoline has a melting point of 19℃. It is a light yellow liquid at room temperature, with the molecular formula of C9H6BrN. It is easily soluble in acetonitrile.

6-Bromoquinoline Synthesis
6-Bromoquinoline was synthesized from 4-bromoaniline,glycerol,nitrobenzene by Skraup reaction.The structure of the title compound was characterized by 1H-NMR.The effect of the oxidant,reaction time and the adjustment of the pH were discussed. The results showed that the highest yield approached 54% when the oxidant was nitrobenzene,refluxing time was 8 h and pH was 5~6.[1]
Analysis and Detection Method of 6-Bromoquinoline
Establish a high-performance liquid chromatography detection method for 6-bromoquinoline,prioritizing C18 column as the separation column. Select trifluoroacetic acid acetonitrile solution and trifluoroacetic acid aqueous solution as mobile phases,and perform gradient elution. The detector is an ultraviolet detector with a detection wavelength of 240nm. Using a Karl Fischer moisture analyzer,study the moisture content of 6-bromoquinoline. Finally, a validation study was conducted on the accuracy and precision of the detection method.[2]
6-Polyamino-substituted quinolines for the detection of metal cations in aqueous media
Chemoselective palladium-catalyzed arylation of polyamines with 6-bromoquinoline has been explored to prepare chelators for the detection of metal cations in aqueous media. The introduction of a single aromatic moiety into non-protected polyamine molecules was achieved using the commercially available Pd(dba)2/BINAP precatalyst to afford nitrogen chelators, in which the aromatic signalling unit is directly attached to the polyamine residue. Water-soluble receptors were then synthesized using N-alkylation of these polyamines by hydrophilic coordinating residues. By combining rich photophysical properties of the 6-aminoquinoline unit with a high coordination affinity of chelating polyamines and a hydrophilic character of carboxamido-substituted phosphonic acid diesters in a single molecular device, we synthesized chemosensor 5 for selective double-channel (UV-vis and fluorescence spectroscopies) detection of CuII ions in aqueous media at physiological levels. This receptor is suitable for the analysis of drinking water and fabrication of paper test strips for the naked-eye detection of CuII ions under UV-light. By increasing the number of donor sites we also obtained chemosensor 6 which is efficient for the detection of HgII ions. Moreover, chemosensor 6 is also suitable for multiple detection of metal ions because it chelates not only HgII but also CuII and ZnII ions displaying different responses of emission in the presence of these three cations.[3]
Incorporation of 6-bromoquinoline into novel chelating ligands
Nitration of 3-bromobenzaldehyde followed by sodium dithionite reduction provides 5-bromo-2-aminobenzaldehyde, which undergoes the Friedländer condensation with a variety of enolizable ketones to afford bidentate and tridentate 6-bromoquinoline derivatives. These species may be dimerized with Ni(0) to form biquinolines or treated under Sonogashira conditions to afford 6-alkynyl derivatives. Examination of optical properties indicate an unusually high emission quantum yield for 6,6'-biquinolines.[4]
Bromo-1,2,3,4-tetrahydroquinolines synthesis
Eight samples of MoS2 were prepared by the hydrothermal reaction of paramolybdates with thiourea where the synthesis temperature was varied from 120 to 180 °C. It was shown by XPS and Raman spectroscopy that the samples mainly consisted of MoS2 but contained significant quantities of oxidized species. All samples had a flower-like morphology, as evidenced by TEM and SEM. The flower-like structure was built of nanosheets aggregated in conglomerates with sizes ranging from 50 nm to ca. 1 μm. The maxima of the quantity vs. size distribution curves of such conglomerates gradually shifted to higher values upon an increase in formation temperatures. All samples were catalytically active in the hydrogenation of quinoline; however, the highest yields of 1,2,3,4-tetrahydroquinoline were achieved for the MoS130-MoS145 samples. The hydrogenation of the isomeric bromo-substituted quinolines in the presence of MoS130-MoS140 was examined. In these cases, the respective bromo-1,2,3,4-tetrahydroquinolines were formed with high selectivity, except for 6-bromoquinoline. The results of the study may be applied to the development of selective catalysts for the hydrogenation of halogen-containing aromatic compounds.[5]
References
[1]. Sun Qb,et al.,.Study on the Synthesis of 6-Bromoquinoline[J].Guangzhou Chemical Industry,2011,39(15):115-116.
[2]. JIao J. Study on 6-Bromoquinoline Analysis and Detection Method[J].Anhui Chemical Industry,2024,50(04):128-130.
[3]. Abel AS, Averin AD, Cheprakov AV, et al. 6-Polyamino-substituted quinolines: synthesis and multiple metal (CuII, HgII and ZnII) monitoring in aqueous media. Org Biomol Chem. 2019;17(17):4243-4260. doi:10.1039/c9ob00259f
[4]. Hu YZ, Zhang G, Thummel RP. Friedl?nder approach for the incorporation of 6-bromoquinoline into novel chelating ligands. Org Lett. 2003;5(13):2251-2253. doi:10.1021/ol034559q
[5]. Terebilenko AV, Olenchuk MV, Mazur DO, et al. Influence of formation temperature on the morphology of MoS2 and its catalytic properties in the hydrogenation of isomeric bromoquinolines to bromo-1,2,3,4-tetrahydroquinolines. Dalton Trans. 2025;54(35):13057-13070. Published 2025 Sep 9. doi:10.1039/d5dt00065c
See also
Lastest Price from 6-Bromoquinoline manufacturers

US $10.00/KG2025-04-21
- CAS:
- 5332-25-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $32.00-266.00/g2025-02-08
- CAS:
- 5332-25-2
- Min. Order:
- 25g
- Purity:
- 0.97
- Supply Ability:
- 25kg