Kuromanin chloride: Synthesis and properties
Introduction
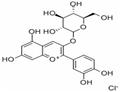
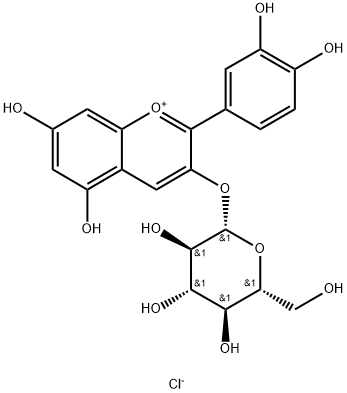
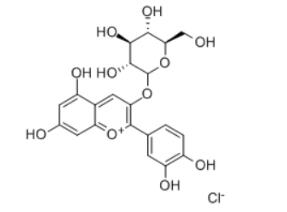
Kuromanin chloride,also kown as cyanidin-3-O-glucoside (Figure 1), one of the most abundant and extensively studied anthocyanins in nature, is a high-value flavonoid compound with diverse physiological activities such as anti-inflammatory, anti-aging, anticancer, antioxidant, and cardiovascular-protective effects. It is widely used in the food, nutraceutical, and cosmetic industries. Currently,commercial kuromanin chloride is primarily extracted from plants, but this approach faces challenges including low yield, high cost, environmental pollution, and difficult purification. Although traditional chemical synthesis has made progress, its industrial application remains limited due to the structural complexity and labor-intensive synthesis of kuromanin chloride.[1]

Synthesis of Kuromanin chloride
Currently, commercial kuromanin chloride is primarily extracted from plants, but this approach faces challenges including low yield, high cost, environmental pollution, and difficult purification. Although traditional chemical synthesis has made progress, its industrial application remains limited due to the structural complexity and labor-intensive synthesis of Kuromanin chloride. With advancements in synthetic biology, microbial cell factories have emerged as a green, economical, and sustainable alternative for natural product production.
Zhang et al. engineered an E. coli cell factory to efficiently convert the low-cost substrate (+)-catechin into high-value kuromanin chloride using genetic engineering,metabolic engineering, and enzyme engineering techniques. This work not only lays the foundation for industrial-scale microbial fermentation of kuromanin chloride but also provides a technical reference for similar research. A kuromanin chloride biosynthetic pathway was successfully established in E. coli BL21(DE3) by introducing anthocyanin synthase (PhANS) from Petunia hybrida and glucosyltransferase (At3GT) from Arabidopsis thaliana, using (+)-catechin as the substrate. To address the limited supply of UDP-glucose, two synthesis pathways utilizing sucrose and cellobiose as substrates were compared. The cellobiose-based pathway proved more efficient, and after optimizing the dual-plasmid copy number, a kuromanin chloride titer of 95.6 mg/L was achieved.[1]
Liu introduced the eriodictyol to catechin synthesis pathway at IS6, IS8, ybbD and recA sites in E.coli BL21(DE3) genome using CRISPR-Cas9 technology, and adjusted the copy number of the pathway genes. Finally, by shaking flask test, it was found that when a copy of the tandem expression box containing F3H, DFR and LAR was introduced at the recA and IS8 loci, the production of catechin was the highest, and the recombinant strain recA 8-2 was obtained. In addition, to obtain the best fermentation parameters, the substrate concentration, the medium of protein expression stage, the concentration of IPTG and the time of adding IPTG in the culture process were optimized. Finally, the highest yield of catechin was 195.5 mg/ when 300 mg/L of the substrate eriodictyol was exogenously added. Furthermore, a recombinant strain recA 82-HpaBC was obtained by introducing the endogenous non-P450 hydroxylase HpaBC from E. coli into recA 8-2, which achieved the synthesis of naringenin to catechin. The highest yield of catechin can reach 28.3 mg/L, and the conversion rate was about 10%. To synthesize kuromanin chloride using the cheaper naringenin as a substrate, a binary co-culture system of E, coli and E. coli was constructed in this study. Finally, using a segmented co-culture strategy, 10.4 mg/L of kuromanin chloride was produced with 300 mg/L naringenin as substrate after 3 h of shaking fermentation.[2]
The effect of Kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma
There is evidence that low doses or physiological concentrations of certain natural polyphenols enhance the activity of telomerase. However, the precise mechanism by which natural polyphenols regulate telomerase activity remains unclear. Recent research indicates that NF-E2 related factor 2 (Nrf2) and silent information regulator 1 (SIRT1) are involved in human telomerase reverse transcriptase (hTERT) regulation. Thus, in order to better comprehend the mechanism by which polyphenols regulate hTERT, the present study investigated the effects of the natural polyphenols Resveratrol, Gallic acid, and Kuromanin chloride on hTERT, Nrf2, and SIRT1 expression as well as oxidative stress in HepG2 hepatocellular carcinoma. The trypan blue dye exclusion assay was used to assess cell viability. The level of mRNA for hTERT, Nrf2, and SIRT1 was then determined using real-time PCR. A spectrophotometric analysis was conducted to quantify oxidative stress markers. Results: The results demonstrated that Resveratrol induces the expression of hTERT and the SIRT1/Nrf2 pathway in a dose-dependent manner. Gallic acid at concentrations of 10 and 20 μM also increased the expression of the hTERT and SIRT1/Nrf2 pathway. Furthermore, dose-dependent overexpression of hTERT and Nrf2 was induced by Kuromanin chloride at 10 and 20 µM. Moreover, we found that Resveratrol and Kuromanin chloride ameliorated oxidative stress, whereas Gallic acid exacerbated it. This study demonstrates that low doses of polyphenols (Resveratrol, Gallic acid, and Kuromanin chloride) upregulate the expression of the hTERT gene in the HepG2 hepatocellular carcinoma cell line, possibly via induction of the SIRT1/Nrf2 signaling pathway. Therefore, by targeting this pathway or hTERT, the anti-cancer effect of polyphenols can be enhanced.[3]
Effect of Kuromanin chloride on senescence of H9C2 cardiomyocytes
To investigate the effect of Kuromanin Chloride on H9c2 cell senescence and its mechanism. D-gal was used to establish a model of H9c2 cardiomyocyte senescence, and the optimal concentration of Kuromanin Chloride was determined. Normal H9c2 cardiomyocytes were divided into normal control group (PBS treatment for 48 h), D-gal treatment group(10 g/L D-gal treatment for 48 h), Kuromanin Chloride treatment group(1 000 μmol/L Kuromanin Chloride treatment for 48 h), and Kuromanin Chloride+D-gal group(co-treatment with 1 000 μmol/L Kuromanin Chloride and 10 g/L D-gal for 48 h). Cell proliferation, cell apoptosis level, and intracellular reactive oxygen species(ROS) level were measured for each group; the SA-β-galactosidase(SA-β-gal) staining kit was used to measure the expression of SA-β-gal; quantitative real-time PCR was used to measure the mRNA expression of CD38, Sirtuin 6(Sirt6), he-xokinase(HK2), lactic acid dehydrogenase A(LDHA), and telomerase reverse transcriptase(TERT); WST-8 assay was used to measure the expression of nicotinamide adenine dinucleotide(NAD+). H9c2 cardiomyocytes were induced by 10 g/L D-gal for 48 h to successfully establish a model of cardiomyocyte senescence, and the optimal concentration of Kuromanin Chloride was determined as 1 000 μmol/L. After intervention with 1 000 μmol/L Kuromanin Chloride, the results showed that Kuromanin Chloride inhibited the reduction in cell proliferation(F=189.50,P<0.01), the increase in cell apoptosis level(F=73.12,P<0.01), the increase in intracellular ROS level(F=106.00,P<0.01), and the increase in the proportion of SA-β-gal-positive cells induced by D-gal in senescent cardiomyocytes, and it also reduced the mRNA expression of CD38, LDHA, and HK2(F=23.14-94.00,P<0.01) and increased the mRNA expression of TERT and Sirt6(F=43.24,86.81;P<0.01) and the level of NAD+(F=77.02,P<0.01) in D-gal-induced senescent cardiomyocytes. Conclusion C3 G can alleviate the senescence of cardiomyocytes by inhibiting the expression of CD38, upregulating the expression of Sirt6, intervening against the apoptosis and proliferation of cardiomyocytes and the change in glycolytic enzyme, and affecting the production of ROS.[4]
References
[1] Zhang YJ. Engineering Escherichia coli for high-efficiency biosynthesis of cyanidin-3-O-glucoside[D].Nanjing Forestry University,2025.DOI:10.27242/d.cnki.gnjlu.2025.000186.
[2] Liu D. Co-culture of Escherichia coli for Synthesis of Cyanidin-3-O-glucoside Using Naringenin as Substrate[D].Shaanxi University of Science and Technology,2024.DOI:10.27290/d.cnki.gxbqc.2024.000421.
[3] Moghadam D, Zarei R, Vakili S, et al. The effect of natural polyphenols Resveratrol, Gallic acid, and Kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma: role of SIRT1/Nrf2 signaling pathway and oxidative stress. Mol Biol Rep. 2023;50(1):77-84. doi:10.1007/s11033-022-08031-7
[4] Zhou HJ,et al.Effect of cyanidin-3-o-glucoside on senescence of H9c2 cardiomyocytes[J].Journal of Qingdao University(Medical Sciences),2022,58(05):708-713.You may like
Related articles And Qustion
Lastest Price from KUROMANIN CHLORIDE manufacturers

US $999.00-666.00/g2025-04-21
- CAS:
- 7084-24-4
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 5000
US $0.00/mg2023-02-24
- CAS:
- 7084-24-4
- Min. Order:
- 5mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g
![10081-67-1 Bis[4-(2-phenyl-2-propyl) phenyl]amineantioxidant KY-4054,4'-bis(α,α-dimethylbenzyl)-diphenylamine](https://www.chemicalbook.com/NewsImg/2019-12-19/201912199545213174.jpg)

![10081-67-1 Bis[4-(2-phenyl-2-propyl) phenyl]amineantioxidant KY-4054,4'-bis(α,α-dimethylbenzyl)-diphenylamine](/NewsImg/2025-11-29/6390003602808618552087063.jpg)