Synephrine: use,mechanism and risk assessment
Introduction
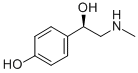
Synephrine(Figure 1) is a molecule present in nature often identified as‘the active component’ of herbal products used for weight-loss,since it is largely found in several vegetable species. Synephrine is also found in the human organism, where it is considered a traceamine due to its low plasmatic levels. Synephrine is the most active substance in Citrus aurantium (CA), the major natural source for the dietary supplements industry. CA is a tree from Rutaceae family popularly named Bitter Orange, Seville Orange, Sour Orange, Green Orange, Zhi Shi, and Kijitsu. The presence of synephrine is also described in another Citrus sp (C. reticulata, C. sinensis, C. deliciosa, C. limon, C.limonia, and C. unshiu) as well as in Evodia rutaecarpa. Synephrine can exist in three different positional isomeric forms (ortho o-, meta m-, and para p-) (Fig. 2) and each positional isomer can also be found in two enantiomeric forms. There is no consensus regarding which synephrine’s positional isomers are present in CA.[1]


General uses of synephrine
Nowadays, obesity is considered a growing public health issue:more than 1 billion adults are overweight [defined by a body mass index (BMI) about 25-29.9 kg/m2] and at least 300 million people are obese (BMI≥30 kg/m2) (WHO, 2009). These situations lead to increased morbidity and mortality due to diseases such as diabetes mellitus, cardiovascular diseases, hypertension, and cancer. Most of the obese and overweight population is medically advised to lose weight.Furthermore, the controversial association between thinness and health or beauty makes individuals with a normal BMI to look for ways to lose weight. Thus, there is a high demand for drugs or dietary supplements in order to obtain anorectic effects by suppressing appetite and food intake or by increasing the energetic metabolism rate through thermogenesis.
People frequently believe that ‘natural products’ are absolutely safe. This erroneous assumption, in addition to the easy access to these over-the-counter products, contributes to the wide popularity of herbal weight-loss products. Among Americans who have consumed dietary supplements, 73.8% used ‘natural products’ containing stimulants such as caffeine, ephedrine, or synephrine.Due to its affinity to a-adrenergic receptors, p-synephrine is marketed as an intravenous drug to treat hypotension. m-Synephrine, also named phenylephrine, has been widely employed in parenteral formulations as an intranasal decongestant, a vasopressor agent used in surgical procedures, in priapism treatment, and as a mydriatic agent. When in 2004, the FDA prohibited the sale of ephedrine-containing dietary supplements because of its strong association with adverse cardiac effects, synephrine (p- and m-) rapidly replaced ephedrine in dietary supplements and became one of the most common compounds used in weight-loss products due to its alleged lipolytic effects.[1]
Mechanism of Synephrine Effects
Synephrine is an adrenergic receptor agonist that acts predominantly through the β-adrenergic receptors (β-ARs). β-ARs are a subfamily of G protein-coupled receptors (GPCRs) that are expressed by most cell types in humans. This subfamily consists of three members, β1, β2, and β3-AR, and are the targets of endogenous catecholamines (epinephrine and norepinephrine). β-Agonists promote receptor-mediated G protein activation of the adenylyl cyclase enzyme and increase cyclic adenosine monophosphate(cAMP) production. Regardless of the subtype differences, all activated β-ARs are typically phosphorylated by regulatory kinases such as GPCR kinases (GRKs), and signaling is then terminated by interaction with β-arrestin. This process is called desensitization.
p-Synephrine and m-synephrine differ in their biological activity due to certain stereochemical differences as separate isomers bind to receptors with different affinity. Therefore, p-synephrine has a lower ability to stimulate a-1, a-2, β-1, and β2-AR compared to the typical sympathomimetics. Moreover, p-synephrine predominantly binds to x1-ARwith lower affinity to a2-AR and thus a much lower potency relative to β-AR, regardless of the subtype. The in vitro studies described above show that the biological effects of psynephrine are mediated by various mechanisms different from selective binding to specific ARs and with a limited contribution of a-, β-1, β-2, and β3-AR. Analysis of the chemical structure and pharmacological action of synephrine indicates that its molecular action is similar and involves interference with the 3',5'-cyclic adenosine monophosphate/proteinkinase A (cAMP/PKA) signaling. This pathway is triggered by sympathetic activation to restore cellular homeostasis via stimulating glucose uptake, lipolysis, fatty acid oxidation,mitochondrial biogenesis, and cell proliferation.[2]
Risk assessment of synephrine in dietary supplements
Synephrine is a sympathomimetic phenylethylamine derivative that occurs naturally in citrus fruits. It is often added to dietary supplements intended for weight loss and enhancement of sports performance, typically in the form of Citrus aurantium extracts and in many cases in combination with caffeine. The health risks of synephrine were evaluated on the basis of the available toxicological data and in accordance to the EFSA guidance on the safety assessment of botanicals and botanical preparations intended for use in food supplements. In animal studies, orally applied synephrine induced adrenergic effects on the cardiovascular system (increase of blood pressure, ventricular arrhythmias), which were enhanced by the concomitant application of caffeine as well as physical activity. Some human intervention studies investigating the acute effects of synephrine on blood pressure and heart rate of healthy, normotensive test persons indicate that synephrine can induce cardiovascular effects in humans. A series of published case reports of adverse cardiovascular effects (hypertension, cardiac arrhythmia, myocardial infarction) were associated with consumption of synephrine- and caffeine-containing dietary supplements. In conclusion, consumption of high amounts of synephrine, especially in combination with caffeine and physical exercise, is associated with an increased risk of adverse effects on the cardiovascular system. According to the assessment by the BfR (Bundesinstitut für Risikobewertung), daily intake of synephrine through dietary supplements should not exceed the median intake from conventional foods.[3]
References
1.Rossato LG, Costa VM, Limberger RP, Bastos Mde L, Remi?o F. Synephrine: from trace concentrations to massive consumption in weight-loss. Food Chem Toxicol. 2011;49(1):8-16. doi:10.1016/j.fct.2010.11.007
2.Dodonova SA, Zhidkova EM, Kryukov AA, et al. Synephrine and Its Derivative Compound A: Common and Specific Biological Effects. Int J Mol Sci. 2023;24(24):17537. Published 2023 Dec 15. doi:10.3390/ijms242417537
3.Bakhyia N, Dusemund B, Richter K, et al. Gesundheitliche Risiken von Synephrin in Nahrungserg?nzungsmitteln [Risk assessment of synephrine in dietary supplements]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60(3):323-331. doi:10.1007/s00103-016-2506-5
You may like
See also
Lastest Price from Synephrine manufacturers

US $0.00/KG2025-04-21
- CAS:
- 94-07-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $10.00/KG2025-04-21
- CAS:
- 94-07-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt