Terephthalic acid:Properties,Mechanism,Application
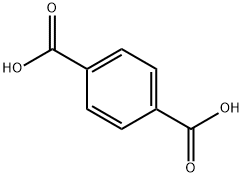
Terephthalic acid is a white solid, it is used principally as a precursor to the polyester PET. The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid.

Properties
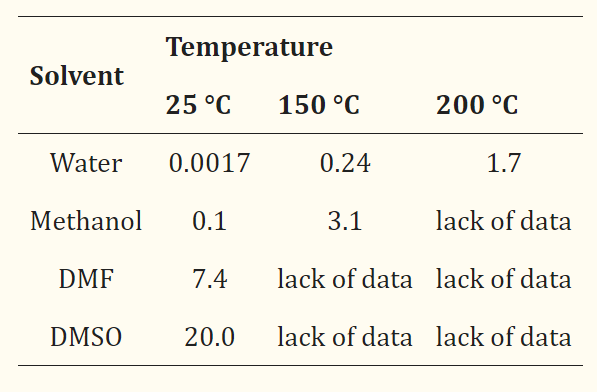
Terephthalic acid is almost insoluble in water, alcohol and ether; it sublimes rather than melting when heated.This insolubility makes it relatively awkward to work with, and up until around 1970 much crude terephthalic acid was converted to the dimethyl ester for purification.
Mechanism
The oxidation of p-xylene proceeds by a free radical process. Bromine radicals decompose cobalt and manganese hydroperoxides. The resulting oxygen-based radicals abstract hydrogen from a methyl group, which have weaker C–H bonds than does the aromatic ring. Many intermediates have been isolated. p-xylene is converted to p-toluic acid, which is less reactive than the p-xylene owing to the influence of the electron-withdrawing carboxylic acid group. Incomplete oxidation produces 4-carboxybenzaldehyde (4-CBA), which is often a problematic impurity.
Application
Virtually the entire world's supply of terephthalic acid and dimethyl terephthalate are consumed as precursors to polyethylene terephthalate (PET). World production in 1970 was around 1.75 million tonnes.By 2006, global purified terephthalic acid (PTA) demand had exceeded 30 million tonnes. A smaller, but nevertheless significant, demand for terephthalic acid exists in the production of polybutylene terephthalate and several other engineering polymers.
Synthesis
The major commercial route to terephthalic acid which is suitable for the direct preparation of poly(ethylene terephthalate) is from p-xylene:
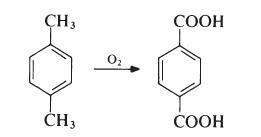
p-Xylene is obtained largely from petroleum sources, being a product of the fractionation of reformed naphthas. The oxidation is carried out in the liquid phase. Typically, air is passed into a solution of p-xylene in acetic acid at about 200℃ and 2 MPa (20 atmospheres) in the presence of a catalyst system containing cobalt and manganese salts and a source of bromide ions. The terephthalic acid produced contains only small amounts of impurities (mainly p-carboxybenzaldehyde), which are readily removed. The acid is dissolved in water at about 2500 e and 5 MPa (50 atmospheres) and treated with hydrogen (which converts the aldehyde to p-toluic acid). The solution is then cooled to 100℃ and pure terephthalic acid crystallizes.
You may like
Related articles And Qustion
See also
Lastest Price from Terephthalic acid manufacturers
US $0.00-0.00/KG2025-12-13
- CAS:
- 100-21-0
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-07-16
- CAS:
- 100-21-0
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month