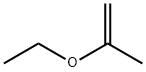
2-Ethoxypropene: Theoretical Study, Application and Bioactivity
Physicochemical property
2-Ethoxypropene is a white to pale yellow solid with a boiling point of 61.2-61.8 °C. It has a density of 0.771.
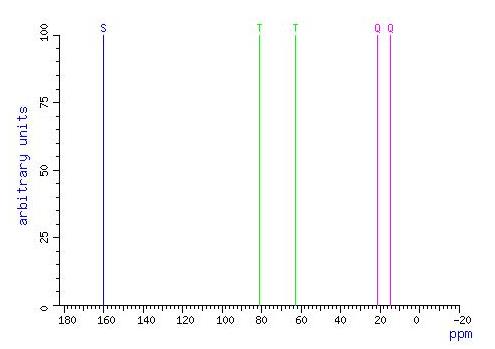
Fig.1 Carbon-13 NMR Spectrum of 2-Ethoxypropene.
Experimental and Theoretical Study
Computational study
This work aims at describing the computational study of thermal decomposition reaction of three oxypropenes: 2-(1-methylethoxy)propene, 2-ethoxypropene, and 2-butoxypropene. It was carried out in order to delve into the reaction mechanism proposed in experimental studies. Computational optimization calculations and frequencies of the structures were performed with the M06-2X/6-311+G(d,p) method at different temperatures ranging from 600 to 640 K. The rate constants were calculated with the classical transition state theory, and the values obtained are of the same order as the reported experimental values. The results derived from the population partitioning technique indicate that transition states are symmetrical and the mechanism is concerted and highly synchronous [1].
Kinetics and Mechanisms
The gas-phase thermal elimination of 2,2-diethoxypropane was found to give ethanol, acetone, and ethylene, while 1,1-diethoxycyclohexane yielded 1-ethoxycyclohexene and ethanol. The kinetics determinations were carried out, with the reaction vessels deactivated with allyl bromide, and the presence of the free radical suppressor cyclohexene and toluene. Temperature and pressure ranges were 240.1-358.3 degrees C and 38-102 Torr. The elimination reactions are homogeneous, unimolecular, and follow a first-order rate law. The rate coefficients are given by the following Arrhenius equations: for 2,2-diethoxypropane, log k(1) (s(-1)) = (13.04 +/- 0.07) - (186.6 +/- 0.8) kJ mol(-1) (2.303RT)(-1); for the intermediate 2-ethoxypropene, log k(1) (s(-1)) = (13.36 +/- 0.33) - (188.8 +/- 3.4) kJ mol(-1) (2.303RT)(-1); and for 1,1-diethoxycyclohexane, log k = (14.02 +/- 0.11) - (176.6 +/- 1.1) kJ mol(-1) (2.303RT)(-1). Theoretical calculations of these reactions using DFT methods B3LYP, MPW1PW91, and PBEPBE, with 6-31G(d,p) and 6-31++G(d,p) basis set, demonstrated that the of 2,2-diethoxypropane and 1,1-diethoxycyclohexane proceeds through a concerted nonsynchronous four-membered cyclic transition state type of mechanism. The rate-determining factor in these reactions is the elongation of the C-O bond. The intermediate product of 2,2-diethoxypropane elimination, that is, 2-ethoxypropene, further decomposes through a concerted cyclic six-membered cyclic transition state mechanism [2].
Rate coefficients at ambient temperature and atmospheric pressure for the reaction of ozone with 2-methoxypropene (2-MPE) and 2-ethoxypropene (2-EPE) were determined in an evacuable 100 L Teflon reaction chamber using absolute and relative rate methods. The product experiments were carried out using a 50 L Teflon reaction chamber in conjunction with FTIR as the detection technique. The rate coefficients (k in units of cm(3) molecule(-1) s(-1)) obtained are 1.18 +/- 0.13 x 10(-17) and 1.89 +/- 0.23 x 10(-17) for reactions with 2-MPE and 2-EPE, respectively. The effects of the alkoxy group on the gas-phase reactivity of alkyl vinyl ethers toward ozone are compared and discussed. The major ozonolysis products are methyl acetate, formaldehyde and CO2 for 2-MPE, and ethyl acetate, formaldehyde and CO2 for 2-EPE. Possible mechanisms for the two vinyl ethers are proposed based on the observed reaction products. Additionally, atmospheric lifetimes of 32 h and 21 h for 2-MPE and 2-EPE were estimated based on the measured rate constants and the ambient tropospheric concentration of ozone, respectively. The obtained values of the lifetimes indicate that the reaction with ozone is an important loss process for these vinyl ethers in the atmosphere, especially in polluted areas [3].
Application
The importance of tunneling effect to the decomposition of 2,2-dimethoxypropane
The experimental results show that the decompositions of 2,2-dimethoxypropane (DMP) and 2,2-diethoxypropane (DEP) have nearly identical rate ratio of 2-methoxypropene (MPP) and 2-ethoxypropene (EPP) in ionic liquids. The yields ratio of MPP/methanol and EPP/ethanol were nearly 1.0. The four-center cyclic transition state was found in B3LYP density functional theory of level. Surprisingly, the experimental observations and theoretical rate constants ratio (k(DEP)/k(DMP)) appeared to differ about ten magnitudes at the experimental temperatures range. Further, dynamics study indicated that the significant tunneling effect played an important role in the identical rate constants ratio of the decomposition reactions at low temperatures range [4].
Improved synthesis of clarithromycin intermediate
2′,4-O-Bis(trimethylsilyl) erythromycin A 9-O-(1-ethoxy-1-methylethyl) oxime(1) is the key intermediate to synthesize clarithromycin,which is one of the most important macrolide antibiotics.With erythromycin A 9-O-oxime used as the starting material,2-ethoxypropene and chlorotrimethylsilane as protection agent,catalized by 1-methyl-2-piperidone hydrochloride,2′,4-O-Bis(trimethylsilyl) erythromycin A 9-O-(1-ethoxy-1-methylethyl) oxime is synthesized in one pot through etherification and silylation.The overall yield is 88%.The superiority of this synthetic process lies in high yield and low pollution owing to the use of 1-methyl-2-piperidone hydrochloride instead of pyridine hydrochloride,and which makes it suitable for industrialized production [5].
Bioactivity
There is still an unmet demand for materials with excellent biocompatibility, controlled hydrolytic capability, and elegant responsiveness to chemical or physical stimuli. To engineer biocompatible materials from beta-cyclodextrin (beta-CD), in this study, we synthesized acetalated beta-CDs (Ac-beta CDs) by one-pot acetalation using 2-ethoxypropene as an acetonation reagent, which can be further processed into nanoparticles (NPs) via the emulsion technique. Ac-beta CD NPs showed pH-labile hydrolysis and pH-triggered release of docetaxel (DTX) payload. Both properties were mainly dominated by the molar ratio of linear to cyclic acetal, which can be conveniently modulated by the acetalation time used for materials synthesis. Ac-beta CD NPs were found to be biocompatible in both in vitro cell culture and in vivo acute toxicity evaluations following intravenous injection. In vitro cell culture experiments demonstrated that antitumor activity of DTX against both sensitive and resistant cancer cells was remarkably improved by formulation into Ac-beta CD nanomedicines. In vivo antitumor study also substantiated the dramatically enhanced efficacy of DTX/Ac-beta CD NPs in a melanoma-bearing nude mouse model. These studies demonstrated that NPs derived from Ac-beta CDs may serve as biocompatible and effective carriers for drug delivery [6].
References
[1] Ruiz P, Quijano J. Computational study of the thermal decomposition of some oxypropenes[J]. Journal of Molecular Modeling, 2020, 26(10): 1-6.
[2] Rosas F, Maldonado A, Lezama J, et al. Kinetics and Mechanisms of the Unimolecular Elimination of 2, 2-Diethoxypropane and 1, 1-Diethoxycyclohexane in the Gas Phase: Experimental and Theoretical Study[J]. The Journal of Physical Chemistry A, 2012, 116(2): 846-854.
[3] Lv C, Du L, Tsona N T, et al. Atmospheric Chemistry of 2-Methoxypropene and 2-Ethoxypropene: Kinetics and Mechanism Study of Reactions with Ozone[J]. Atmosphere, 2018, 9(10): 401.
[4] Jiang H, Li H, Wu T, et al. The importance of tunneling effect to the decomposition of 2, 2-dimethoxypropane and 2, 2-diethoxypropane[J]. Chemical physics letters, 2005, 406(4-6)
You may like
Related articles And Qustion
See also
Lastest Price from 2-Ethoxypropene manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 926-66-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $5.00-2.00/KG2024-10-11
- CAS:
- 926-66-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg