Chebulagic acid: Preparation and Bioactivity
General description
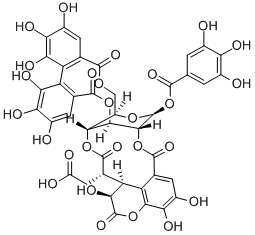
Chebulagic acid is also called chekhotannin. Tannin is a class of polyphenolic compounds, which can be divided into hydrolyzed tannin and condensed tannin. Hydrolyzed tannin has good pharmacological effects such as anti-oxidation, anti-tumor, anti-virus, antibacterial and anti-lipid peroxidation, and is the basis of pharmacodynamic substances in many common Chinese medicines.
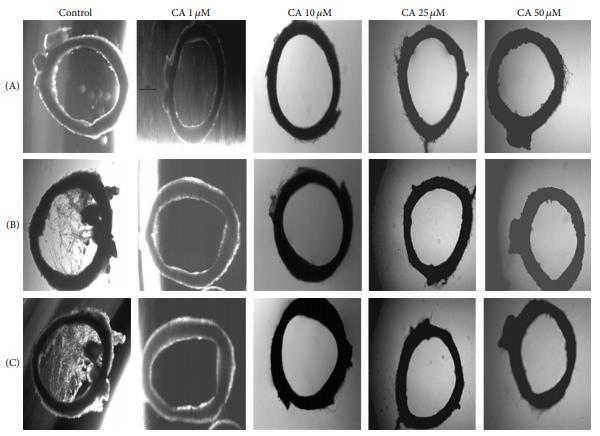
Fig. 1. Effect of chebulagic acid on sprouting of rat aortic rings. Rat aortic ring explants were maintained in culture in the presence of 10% fetal bovine serum (FBS). Different concentrations of chebulagic acid (1 ??M, 10 ??M, 25 ??M, and 50 ??M) were supplemented to the medium and was monitored sprouting, under a microscope at different time intervals. The morphological changes were visualized and photographed under a microscope (×4) on the 1st (A), 3rd (B), and 5th (C) days. Each set was done in replicates, and the microphotographs from a representative experiment are given.
Preparation
Chebulagic acid is a hydrolyzable tannin found in the fruits of Terminalia bellirica and has been extensively used in traditional Indian medicine. Currently, most of the chebulagic acid is extracted from Terminalia chebula and a lesser extent, from T. bellirica. Crude chebulagic acid extracts from these fruits are usually extracted using Soxhlet extraction or reflux methods before purification using column chromatography and High-Performance Liquid Chromatography (HPLC). We examined supercritical fluid extraction efficiency with or without a modifier in extracting chebulagic acid from T. bellirica fruit powder in the present study. Our results demonstrated that supercritical fluid extraction with 50% ethanol as a modifier (SFEM) was the most efficient method in extracting chebulagic acid from T. bellirica fruit powder compared to supercritical fluid extraction without modifier (SFE) and Soxhlet extraction (SE) methods. This new extraction method (SFEM) yielded 96.10% +/- 3.34 (w/w) chebulagic acid with 96.9% +/- 1.34 purity and 95.21% +/- 0.14 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity, requiring no further purification. HPLC was used to confirm the purity of chebulagic acid and the chemical structure was confirmed using their mass spectroscopy (MS) and H-1 nuclear magnetic resonance (NMR) spectra. This one-step extraction and purification method is clean, uses less liquid solvent compared to the conventional Soxhlet method, and the extraction process requires 92% less time. Novelty impact statement In this current study, the supercritical fluid extraction method yielded 96.10% +/- 3.34 (w/w) chebulagic acid with 96.9% +/- 1.34 purity and required no further purification. In addition, this one-step extraction and purification method is clean, uses less liquid solvent compared to the conventional Soxhlet method, and the extraction process requires 92% less time [1].
Bioactivity
Anti-Hyperglycemic
In the present study, we firstly compared rat intestinal alpha-glucosidase inhibitory activity by different ethanol-aqueous extractions from the dried fruits of Terminalia chebula Retz. The enzymatic assay showed that the 80% ethanol extract was more potent against maltase activity than both 50% and 100% ethanol extracts. By HPLC analysis, it was determined that the 80% ethanol extract had a higher content of chebulagic acid than each of 50% or 100% ethanol extract. Next, we investigated how efficiently chebulagic acid could inhibit sugar digestion by determining the glucose level on the apical side of the Caco-2 cell monolayer. The result showed that the maltose-hydrolysis activity was down-regulated by chebulagic acid, which proved to be a reversible inhibitor of maltase in Caco-2 cells. On the other hand, chebulagic acid showed a weak inhibition of sucrose-hydrolysis activity. Meanwhile, chebulagic acid did not have an obvious influence on intestinal glucose uptake and was not effective on glucose transporters. Further animal studies revealed that the oral administration of chebulagic acid (100 mg/kg body weight) significantly reduced postprandial blood glucose levels by 11.1% in maltose-loaded Sprague-Dawley (SD) rats compared with the control group, whereas the oral administration of chebulagic acid did not show a suppressive effect on postprandial hyperglycemia in sucrose-or glucose-loaded SD-rats. The results presented here suggest that chebulagic acid from T. chebula can be used to control blood glucose and manage type 2 diabetes, although clinical trials are needed [2].
Ferroptosis-Inhibitory
The search for a safe and effective inhibitor of ferroptosis, a recently described cell death pathway, has attracted increasing interest from scientists. Two hydrolyzable tannins, chebulagic acid and chebulinic acid, were selected for the study. Their optimized conformations were calculated using computational chemistry at the B3LYP-D3(BJ)/6-31G and B3LYP-D3(BJ)/6-311 + G(d,p) levels. The results suggested that (1) chebulagic acid presented a chair conformation, while chebulinic acid presented a skew-boat conformation; (2) the formation of chebulagic acid requires 762.1729 kcal/mol more molecular energy than chebulinic acid; and (3) the 3,6-HHDP (hexahydroxydiphenoyl) moiety was shown to be in an (R)- absolute stereoconfiguration. Subsequently, the ferroptosis inhibition of both tannins was determined using a erastin-treated bone marrow-derived mesenchymal stem cells (bmMSCs) model and compared to that of ferrostatin-1 (Fer-1). The relative inhibitory levels decreased in the following order: Fer-1 > chebulagic acid > chebulinic acid, as also revealed by the in vitro antioxidant assays. The UHPLC-ESI-Q-TOF-MS analysis suggested that, when treated with 16-(2-(14-carboxytetradecyl)-2-ethyl-4,4-dimethyl-3-oxazolidinyloxy free radicals, Fer-1 generated dimeric products, whereas the two acids did not. In conclusion, two hydrolyzable tannins, chebulagic acid and chebulinic acid, can act as natural ferroptosis inhibitors. Their ferroptosis inhibition is mediated by regular antioxidant pathways (ROS scavenging and iron chelation), rather than the redox-based catalytic recycling pathway exhibited by Fer-1. Through antioxidant pathways, the HHDP moiety in chebulagic acid enables ferroptosis-inhibitory action of hydrolyzable tannins [3].
Neuroprotective Effect
Autophagy is a series of catabolic process mediating the bulk degradation of intracellular proteins and organelles through formation of a double-membrane vesicle, known as an autophagosome, and fusing with lysosome.