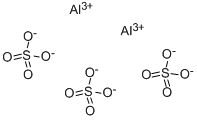
Chemical Properties
Aluminium sulfate is a colorless or white crystals. Odorless, slightly sweet taste. Because of containing iron etc, industrial product looks like a yellowish-green, tastes sour. It was Stable in the air, heated to 250 ℃ to lose crystal water, when heated above 700 ℃, begin decompose into aluminum oxide, sulfur trioxide and water vapor, etc. Soluble in water, aqueous solution is acidic.

Uses
Aluminium sulfate is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.
Production Methods
Aluminium sulfate may be made by adding aluminium hydroxide, Al(OH)3, to sulfuric acid, H2SO4:
2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6 H2O
or by heating aluminium metal in a sulfuric acid solution
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2↑
Health Hazard
ADI not specified (FAO/WHO, 2001).
GRAS (FDA, §182.1125,2000).
LD50 6207mg/kg (mice, by mouth)
Chemical Reactivity
Aluminium sulfate reacts with sodium bicarbonate to which foam stabilizer has been added, producing carbon dioxide for fire-extinguishing foams:
Al2(SO4)3 + 6 NaHCO3 → 3 Na2SO4 + 2 Al(OH)3 + 6 CO2
The carbon dioxide is trapped by the foam stabilizer and creates a thick foam which will float on top of hydrocarbon fuels and seal off access to atmospheric oxygen, smothering the fire.
Toxicity
ADI not specified (FAO/WHO, 2001).
GRAS (FDA, §182.1125,2000).
LD50 6207mg/kg (mice, by mouth)
Toxic to aquatic life
Source
Aluminium sulfate occurs in nature in minerals; alunite, KAl3(SO4)2(OH)6and natroalunite, NaAl3(SO4)2(OH)6. The anhydrous salt is used in food applications.