Description
Indigo carmine is indigotindisulfonate sodium, a marker dye used during cystoscopy and ureteral catheterization.
(1) Indigo carmine is also used in endoscopic procedures, lymph node and vessel delineation, and for tumor localization. Indigo carmine is an unapproved drug without FDA approved labeling.
(2) There is no single dye that can replace indigo carmine. Choice of alternative agent will depend on type of procedure and physician discretion, as well as product availability.
Indigo Carmine (IC) is the most common chemical dye for clothes dyeing, which is considered as a refractory molecule since it is required a rather strong chemical treatment for its elimination from the water waste. The presence of this dye in residuals causes notorious change of water color and smell even in very low concentration. Moreover, if some of this water reaches natural streams it can be toxic for aquatic living entities due to the formation of toxic compounds such as aromatic amines.
Chemical Properties
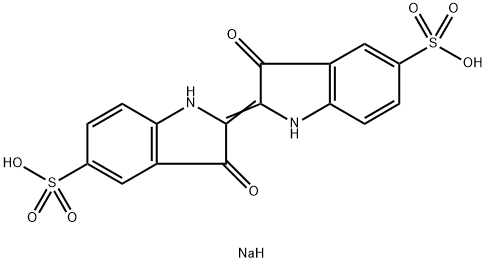
FD & C Blue No. 2 is principally the disodium salt of 2-( 1,3-dihydro-3-oxo-5-sulfo-2H-indol-2-ylidene)-2,3-dihydro3-oxo-lH-indole-5-sulfonic acid.
The colorant is a blue-brown to red-brown powder that dissolves in water to give a solution that is blue at neutrality, blue-violet in acid, and green to yellow-green in base. When dissolved in concentrated sulfuric acid, it yields a blue-violet solution that turns blue when diluted with water. It is soluble in 95% ethanol.
FD & C Blue No. 2 is used in dessert powders, bakery goods, cereals, snack foods, confectionery products, maraschino cherries, sausage, ice cream, sherbet, and dairy products. The color is also used in drug preparations.1
.
Chemical Properties
purple powder
Uses
Indigo Carmine (FD&C Blue #2) is a colorant. It has poor ph stability in that after 1 week at ph 3 and 5 it will appreciably fade, at ph 7 considerably fade, and at ph 8 fade completely. It is the least soluble of all food colors, with a solubility of 1.6 g in 100 ml of water at 25°c. Complete fading occurs in alkalis such as 10% sodium carbonate and 10% sodium hydroxide, with fading also occurring in 10% sugar systems. It has very poor light stability and oxidation stability, and moderate stability to heat; it has a deep blue hue with poor tinctorial strength. It is the only food color that has good resistance to reducing agents, but has very poor compatibility with food components. The major use is in pet food, but it is also used in candies, confections, and baked goods. The common name is indigotine.
Uses
5,5’Indigodisulfonic Acid Disodium Salt is a food additive that is widely used in the market. 5,5’Indigodisulfonic Acid Disodium Salt was also used as a dye for cationic cotton fabrics.
Uses
Colorant for nylon, surgical sutures, foods and ingested drugs. As a reagent for functional kidney tests, for detection of nitrates, chlorates and in testing milk.
Preparation
3H-indol-3-one,2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro?with strong sulfuric acid or trace fuming sulfuric acid products.
Definition
ChEBI: An organic sodium salt resulting from the formal condensation of indigo carmine (acid form) with two equivalents of sodium hydroxide. It is an indicator at pH 11.5-14, changing from blue to yellow.
brand name
Indigo Carmine (Becton Dickinson Microbiology).
General Description
Indigo to dark blue powder.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sensitive to light. Very sensitive to oxidizing agents. Color is readily discharged by nitric acid, chlorates, etc. Color of aqueous solutions fades on standing.
Fire Hazard
Flash point data for Acid Blue 74 is not available, but Acid Blue 74 is probably combustible.
Biochem/physiol Actions
Indigo Carmine (IC), commonly known as acid blue 74 or food blue 1 or FD and C blue 2 is a blue synthetic dye. It is used as a redox indicator in analytical chemistry and as a microscopic stain in biology. IC is also employed as a photometric detector. IC is a highly toxic indigoid dye and is associated with various health hazards such as irritation to the gastrointestinal tract leading to nausea, vomiting, and diarrhea and irritation in the respiratory tract leading to coughing and shortness of breath. IC exposure might also cause skin and eye irritations to humans. IC dye spray is used for the diagnosis of dysplasia in ulcerative colitis and is considered to be a simple, feasible, and safe method.
Properties and Applications
green light blue. Soluble in water for blue, slightly soluble in alcohol. The strong sulfuric acid is a deep blue light in purple, diluted into blue. In aqueous solution for green to yellow sodium hydroxide to join.
|
Standard
|
Light Fastness
|
Soaping
|
Persperation Fastness
|
Oxygen bleaching
|
Fastness to seawater
|
|
Fading
|
Stain
|
Fading
|
Stain
|
Fading
|
Stain
|
|
ISO
|
1-2
|
2
|
4
|
2
|
1
|
2
|
2
|
1
|
|
AATCC
|
1
|
3
|
5
|
1
|
1
|
1
|
2
|
2
|
Purification Methods
Its solubility in H2O is 1g/100mL at 25o. It has been purified by dissolving in H2O, filtering and adding EtOH to cause the salt to separate. Wash the solid with EtOH, Et2O and dry in vacuo. [V.rlander & Schubert Chem Ber 34 1860 1901, UV: Smit et al. Anal Chem 27 1159 1955, Preisler et al. J Am Chem Soc 81 1991 1959, Beilstein 25 IV 1975.]
References
American Society of Health-system Pharmacist.
Journal of Environmental Protection, 2016, 7, 1693-1706