Description
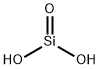
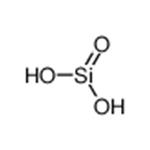
Silicic acid is the general name that refers to the group of chemical compounds of the element hydrogen, silicon, and oxygen with the formula [SiOx(OH)4-2x]n. H4SiO4 is the most predominant form.
Silicic acid is a hydrated form of silicon dioxide. It belongs to the family of Miscellaneous Silicates. These are inorganic compounds in which the largest metallic oxoanion is silicate, to which either no atom or a non metal atom is bonded.
Silicic acid is contained in many foods, such as grain, fruit and vegetables (borage). It is commonly used in the manufacture of toothpastes and as a stationary phase for chromatography.It is utilized for the manufacture catalyst and catalyst carrier. It is involved in tungsten filament production as chemical reagent and flux. Further, it is used for oil and wax decoloring.
Chemical Properties
Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder
Uses
Laboratory reagent and reinforcing agent in
rubber.
Uses
Silicic acid is used in protein chromatography. Silicic acid has been used in a study to demonstrate the anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of
Serratia marcescens. Silicic acid has also been used to study the antioxidant activity of fresh and processed Jalapeno and Serrano peppers.
Definition
The jellylike precipitate obtained when sodium
silicate solution is acidified. The proportion of
water varies with the conditions of preparation
and decreases gradually during drying and ignition,
until relatively pure silica remains. During drying
the je
Biochem/physiol Actions
Silicic acid polymers are employed in water treatment systems, drug encapsulation cum delivery and for soluble nanoparticle generation. Silicic acid interacts with aluminum and reduces its bioavailability. It is an effective antidote for aluminum poisoning.
Safety Profile
An inhalation hazard. Poison by intravenous route. An eye irritant and nuisance dust. Questionable carcinogen. Mutation data reported. See also other silica entries and SILICATES
Potential Exposure
Amorphous fumed silica is used as a mineral, natural or synthetic fiber. A potential danger to those involved in the production and handling of fumed silica for paint pigments or catalysts. Diatomaceous earth is used in clarifying liquids, in manufacture of fire brick and heat insulators; used as a filtering agent; as a filler in construction materials; pesticides, paints, and varnishes. A potential danger to those involved in mining of diatomaceous earth or fabrication of products there from.
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Waste Disposal
Sanitary landfill.