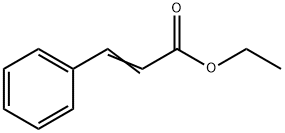
Ethyl cinnamate
- Product NameEthyl cinnamate
- CAS103-36-6
- CBNumberCB9226049
- MFC11H12O2
- MW176.21
- EINECS203-104-6
- MDL NumberMFCD00009189
- MOL File103-36-6.mol
Chemical Properties
| Melting point | 6-8 °C (lit.) |
| Boiling point | 271 °C (lit.) |
| Density | 1.049 g/mL at 20 °C (lit.) |
| vapor pressure | 6Pa at 20℃ |
| FEMA | 2430 | ETHYL CINNAMATE |
| refractive index | n |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Odor | at 100.00 %. sweet balsam fruity spicy powdery berry plum |
| Odor Type | balsamic |
| biological source | synthetic |
| Water Solubility | insoluble |
| Merck | 14,2299 |
| JECFA Number | 659 |
| BRN | 1238804 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H303 | |||||||||
| Precautionary statements | P270-P301+P312-P403-P501c | |||||||||
| Risk Statements | 20-22 | |||||||||
| Safety Statements | 23-24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GD9010000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29163990 | |||||||||
| Toxicity | The acute oral LD50 value in rats was reported as 7.8 g/kg (7.41-8.19 g/kg) (Russell, 1973). The acute dermal LD50 value in rabbits was reported as > 5 g/kg (Russell, 1973). | |||||||||
| NFPA 704: |
|


