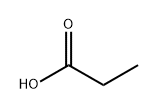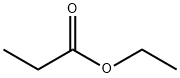
Ethyl propionate
- Product NameEthyl propionate
- CAS105-37-3
- CBNumberCB3240869
- MFC5H10O2
- MW102.13
- EINECS203-291-4
- MDL NumberMFCD00009308
- MOL File105-37-3.mol
- MSDS FileSDS
Chemical Properties
| Melting point | -73 °C (lit.) |
| Boiling point | 99 °C (lit.) |
| Density | 0.888 g/mL at 25 °C (lit.) |
| vapor density | 3.52 (vs air) |
| vapor pressure | 40 mm Hg ( 27.2 °C) |
| FEMA | 2456 | ETHYL PROPIONATE |
| refractive index | n |
| Flash point | 54 °F |
| storage temp. | Flammables area |
| solubility | 2% soluble in water. miscible with alcohol, perfume and flavor oils. |
| form | Liquid |
| color | Clear colorless to pale yellow |
| PH | 7 (H2O, 20℃) |
| Odor | at 10.00 % in dipropylene glycol. sweet fruity rum juicy fruit grape pineapple |
| Odor Type | fruity |
| Odor Threshold | 0.007ppm |
| biological source | synthetic |
| explosive limit | 1.8-11%(V) |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H225-H315-H319-H335 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | F | |||||||||
| Risk Statements | 11 | |||||||||
| Safety Statements | 16-23-24-29-33 | |||||||||
| RIDADR | UN 1195 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | UF3675000 | |||||||||
| Autoignition Temperature | 887 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29159000 | |||||||||
| Hazardous Substances Data | 105-37-3(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|