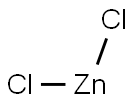
Zinkchlorid
Bezeichnung:Zinkchlorid
CAS-Nr7646-85-7
Englisch Name:Zinc chloride
CBNumberCB4854265
SummenformelCl2Zn
Molgewicht136.3
MOL-Datei7646-85-7.mol
Synonyma
Zinkchlorid
Zinkdichlorid
Salzsaures Zink
Zinkchlorid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 293 °C (lit.) |
| Siedepunkt | 732 °C (lit.) |
| Dichte | 1.01 g/mL at 20 °C |
| chüttdichte | 1400-1800kg/m3 |
| Dampfdruck | 1 mm Hg ( 428 °C) |
| Flammpunkt | 732°C |
| storage temp. | 2-8°C |
| Löslichkeit | H2O: 4 M at 20 °C, clear, colorless |
| pka | pKa 6.06 (Uncertain) |
| Aggregatzustand | crystalline |
| Wichte | 2.91 |
| Farbe | white |
| PH | 5 (100g/l, H2O, 20℃) |
| Geruch (Odor) | wh. cubic cryst., odorless |
| Wasserlöslichkeit | 432 g/100 mL (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,10132 |
| crystal system | square |
| Kennzeichnung gefährlicher | Xi,N,C,F+,F,Xn |
| R-Sätze: | 36/37/38-50/53-34-22-51/53-67-66-19-12-11-40 |
| S-Sätze: | 26-36-61-60-45-36/37/39-16-36/37 |
| RIDADR | UN 2924 3/PG 1 |
| OEB | C |
| OEL | TWA: 1 mg/m3, STEL: 2 mg/m3 |
| WGK Germany | 2 |
| RTECS-Nr. | TY2900000 |
| F | 3 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | I |
| HS Code | 28273600 |
| Giftige Stoffe Daten | 7646-85-7(Hazardous Substances Data) |
| Toxizität | Inhalation of zinc chloride fumes can injure lungs and respiratory tract. Dusts or fumes also cause dermatitis, boils, conjunctivitis, and gastrointestinal tract upset (Lewis(Sr), R.J. 1996. Sax’s Dangerous Properties of Industrial Materials, 9th ed. New York: Van Nostrand Reinhold). LD50 oral (rat): 350mg/kg LCLO (inhalation): 1.960 g/m3/10 min |
| IDLA | 50 mg/m3 |



