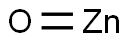
Zinkoxid
Bezeichnung:Zinkoxid
CAS-Nr1314-13-2
Englisch Name:Zinc oxide
CBNumberCB3853034
SummenformelOZn
Molgewicht81.39
MOL-Datei1314-13-2.mol
Synonyma
Zinkoxid
Zinkwei?
Zinkmonoxid
Zinkoxid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 1975 °C |
| Siedepunkt | 1949.9°C (estimate) |
| Dichte | 5.6 |
| chüttdichte | 200-700kg/m3 |
| Brechungsindex | 2.008~2.029 |
| Flammpunkt | 27℃ |
| storage temp. | Store at +5°C to +30°C. |
| Löslichkeit | 0.0016g/l insoluble |
| Aggregatzustand | nanopowder |
| Wichte | 5.61 |
| Farbe | White to pale yellow |
| PH | 7 (50g/l, H2O, 20℃)(slurry) |
| Geruch (Odor) | wh. to gray powd. or crystals, odorless, bitter taste |
| Wasserlöslichkeit | 1.6 mg/L (29 ºC) |
| Crystal Structure | ZnO type |
| crystal system | Six sides |
| Merck | 14,10147 |
| Space group | P63mc |
| Kennzeichnung gefährlicher | N,Xn,F |
| R-Sätze: | 50/53-20-43-36/38-20/21/22-67-66-10-11 |
| S-Sätze: | 60-61-7/9-36/37-26-16 |
| RIDADR | UN 3077 9/PG 3 |
| OEB | B |
| OEL | TWA: 5 mg/m3, Ceiling: 15 mg/m3 |
| WGK Germany | 2 |
| RTECS-Nr. | ZH4810000 |
| TSCA | Yes |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 28170000 |
| Giftige Stoffe Daten | 1314-13-2(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: > 5000 mg/kg |
| IDLA | 500 mg/m3 |



