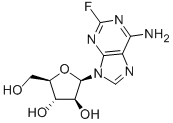
Флударабин
- английское имяFludarabine
- CAS №21679-14-1
- CBNumberCB8188247
- ФормулаC10H12FN5O4
- мольный вес285.23
- EINECS244-525-5
- номер MDLMFCD00132942
- файл Mol21679-14-1.mol
| Температура плавления | 265-268°C |
| альфа | D25 +17 ±2.5° (c = 0.1 in ethanol) |
| Температура кипения | 747.3±70.0 °C(Predicted) |
| плотность | 2.17±0.1 g/cm3(Predicted) |
| температура хранения | 2-8°C |
| растворимость | DMF: 20 mg/mL, clear, faintly yellow |
| пка | 13.05±0.70(Predicted) |
| форма | Powder |
| цвет | White to Pale Yellow |
| Растворимость в воде | Soluble in DMF, DMSO, methanol or ethanol. Sparingly soluble in water |
| Мерк | 13,4152 |
| БРН | 1225932 |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| InChI | InChI=1/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6+,9-/s3 |
| ИнЧИКей | HBUBKKRHXORPQB-VOSPVXAENA-N |
| SMILES | C12N=C(N=C(N)C=1N=CN2[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1)F |&1:10,11,13,15,r| |
| Справочник по базе данных CAS | 21679-14-1(CAS DataBase Reference) |
| FDA UNII | P2K93U8740 |
| Код УВД | L01BB05 |
| UNSPSC Code | 12352202 |
| NACRES | NA.77 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 23/24/25-36/37/38-39/23/24/25-39-22 | |||||||||
| Заявления о безопасности | 26-36/37-45-36 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AU6207000 | |||||||||
| F | 10 | |||||||||
| кода HS | 29349990 | |||||||||
| Банк данных об опасных веществах | 21679-14-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Флударабин химические свойства, назначение, производство
Химические свойства
White SolidИспользование
Fludarabine is not one of the most used drugs in pediatric oncology area. It is used in combination with other drugs to treat AML in children, mostly those who are receiving second-line therapy. It is commonly sold as 10 mg film-coated tablets and IV vial containing 50mg.Показания
Fludarabine (Fludara) is a fluorinated purine analogue of the antiviral agent vidarabine.The active metabolite, 2-fluoro-ara-adenosine triphosphate, inhibits various enzymes involved in DNA synthesis, including DNA polymerase-α, ribonucleotide reductase, and DNA primase. Unlike most antimetabolites, it is toxic to nonproliferating as well as dividing cells, primarily lymphocytes and lymphoid cancer cells.The drug is highly active in the treatment of chronic lymphocytic leukemia, with approximately 40% of patients achieving remissions after previous therapy with alkylating agents has failed. Activity is also seen in the low-grade lymphomas.
The major side effect is myelosuppression, which contributes to fevers and infections in as many as half of treated patients. Nausea and vomiting are mild. Occasional neurotoxicity has been noted at higher doses, with agitation, confusion, and visual disturbances.
Общее описание
The drug is available as the phosphate salt in a 50-mg vialfor IV use. Fludarabine is used to treat chronic lymphocyticleukemia and non-Hodgkin’s lymphoma. The mechanism ofaction involves the triphosphate metabolite and its inhibitionof DNA chain elongation. The 2-fluoro group on the adeninering renders fludarabine resistant to breakdown byadenosine deaminase. The drug is rapidly dephosphorylatedto 2-fluoro-ara-adenosine (F-ara-A) after administration. Fara-A is taken into the cell and subsequently re-phosphorylatedto yield the triphosphate (F-ara-ATP), the active drugspecies. Resistance can occur via decreased expression ofthe activating enzymes and decreased drug transport.Fludarabine is orally bioavailable and is distributed throughoutthe body reaching high levels in liver, kidney, andspleen. The drug is metabolized to F-ara-A, which enterscells via the nucleoside transport system and is rephosphorylatedby deoxycytidine kinase to fludarabine monophosphateand finally fludarabine triphosphate, the activespecies. About 25% of F-ara-A is excreted unchanged inurine. Drug interactions include an increased incidence offatal pulmonary toxicity when fludarabine is used in combinationwith pentostatin. Additionally, fludarabine may potentiate the effects of several other anticancer drugs includingcytarabine, cyclophosphamide, and cisplatin.Toxicities include myelosuppression, immunosuppression,fever, nausea, and vomiting.Биологическая активность
Purine analog that inhibits DNA synthesis. Exhibits antiproliferative activity (IC 50 = 1.54 μ M in RPMI cells) and triggers apoptosis through increasing Bax and decreasing Bid, XIAP and survivin expression. Displays anticancer activity against hematological malignancies in vivo .Способ действия
Fludarabine is a fluorinated analogue of adenine that is relatively resistant to deamination by adenosine deaminase. Fludarabine phosphate is a prodrug that is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active metabolite triphosphate 2-fluoro-ara-ATP. Fludarabine inhibits the DNA synthesis via inhibition of ribonucleotide reductase, DNA polymerase (α, δ, and ε), DNA primase, and DNA ligase. The action mechanism also is by partial inhibition of RNA polymerase II, causing reduction in protein synthesis. It is believed that effects on DNA, RNA, and protein synthesis contribute to the inhibition of cell growth, mostly by inhibition of DNA synthesis. Lymphocytes of CLL when exposed, in vitro, to the compound 2-fluoro-ara-A lead to extensive DNA fragmentation and apoptosis.Флударабин запасные части и сырье
Флударабин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18958038633; +8618958038633 |
China | 64 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 |