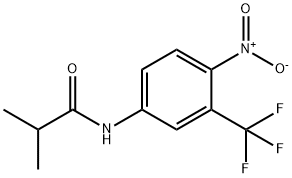
Флутамид
- английское имяFlutamide
- CAS №13311-84-7
- CBNumberCB2468110
- ФормулаC11H11F3N2O3
- мольный вес276.21
- EINECS236-341-9
- номер MDLMFCD00072009
- файл Mol13311-84-7.mol
| Температура плавления | 112 °C |
| Температура кипения | 400.3±45.0 °C(Predicted) |
| плотность | 1.3649 (estimate) |
| температура хранения | 2-8°C |
| растворимость | Practically insoluble in water, freely soluble in acetone and in ethanol (96 per cent). |
| пка | 13.12±0.70(Predicted) |
| форма | Solid |
| цвет | Pale Yellow to Light Yellow |
| Мерк | 14,4208 |
| InChI | InChI=1S/C11H11F3N2O3/c1-6(2)10(17)15-7-3-4-9(16(18)19)8(5-7)11(12,13)14/h3-6H,1-2H3,(H,15,17) |
| ИнЧИКей | MKXKFYHWDHIYRV-UHFFFAOYSA-N |
| SMILES | C(NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1)(=O)C(C)C |
| Справочник по базе данных CAS | 13311-84-7(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | flutamide |
| FDA UNII | 76W6J0943E |
| Словарь наркотиков NCI | flutamide |
| Код УВД | L02BB01 |
| Предложение 65 Список | Flutamide |
| Система регистрации веществ EPA | Flutamide (13311-84-7) |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 20/21/22-63-36/37/38 | |||||||||
| Заявления о безопасности | 22-36-36/37/39-27-26 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | UG5700000 | |||||||||
| кода HS | 29242990 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H411:Токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H361:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Флутамид химические свойства, назначение, производство
Описание
Flutamide is an orally active, non-steroidal antiandrogen indicated for the treatment of prostatic cancer in both castrates and noncastrates.Химические свойства
Light Yellow SolidИстория
Flutamide was first described as a member of a series of N-acyl anilides synthesized at Monsanto in the 1960s during a compound finding program aiming at bacteriostatic agents. Soon after, at Schering Corp., the compound was characterized pharmacologically and further developed as SCH-13521. It was found that flutamide inhibits agonist action at the AR by replacing the agonist at the ligand binding site, being the first nonsteroidal compound possessing anti-androgenic activity in animals. In contrast to steroidal anti-androgens, for instance, cyproterone acetate, which also displays significant progestational activity, flutamide has no other hormonal activity. There is also no reduction of serum testosterone levels seen with flutamide but rather a slight increase in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) resulting in elevated serum testosterone levels. This accounts for the beneficial maintenance of libido and potency in sexually active patients. On the other hand, elevated serum estradiol levels resulting from peripheral testosterone aromatization leading to gynecomastia were observed in patients.Использование
Flutamide is a nonsteroidal antiandrogen drug; antineoplastic (hormonal).Показания
Flutamide (Eulexin) is a nonsteroidal androgen receptor antagonist that inhibits androgen binding to its nuclear receptor. It is effective in inducing prostatic regression and is approved for the treatment of prostatic carcinoma. For maximum clinical effectiveness it has to be used in combination with a GnRH antagonist (e.g., leuprolide acetate) that inhibits androgen production. Flutamide may eventually be used for the treatment of hirsutism and male pattern baldness in women if a topical preparation is developed.Общее описание
Flutamide, 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide, is dosed 3 times daily(250-mg dose; 750-mg total daily dose). A major metaboliteof flutamide, hydroxyflutamide, is a more potent AR antagonistthan the parent compound. This metabolite, which ispresent at a much higher steady-state concentration than isflutamide, contributes a significant amount of the antiandrogen action of this drug. A limiting factor in the useof flutamide is hepatotoxicity in from 1% to 5% of patients.Although the hepatotoxicity usually is reversible followingcessation of treatment, rare cases of death associated withhepatic failure have been reported to be associated with flutamidetherapy. Diarrhea is also a limiting side effect withflutamide therapy for some patients.Контактные аллергены
Flutamide is an antiandrogenic hormonal antineoplas tic drug that can induce photosensitivity and porphy ria-like eruption.Механизм действия
Flutamide is a nonsteroid drug that possesses antiandrogenic action. It blocks androgens from binding with target tissues, thus preventing androgen action. The mechanism of action is possibly also linked with a halt in dihydrotestosterone transport. It facilitates a reduction in size and density of the prostate gland, and it reduces the amount of metastases in such cancer, for which it is used in palliative treatment of prostate gland cancer.Клиническое использование
Flutamide is a pure antagonist, whereas 2-hydroxyflutamide is a more potent AR antagonist but also can activate the androgenic receptor at higher concentrations. These findings raise the possibility that increased conversion of flutamide to 2-hydroxyflutamide or accumulation of 2-hydroxyflutamide in cells may contribute to the anomalous responses to flutamide that are observed in some advanced prostate cancers.Флутамид запасные части и сырье
Флутамид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618058761490 | China | 49983 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +8615102730682 | CHINA | 566 | 55 | ||
| 025-85710122 17714198479 | CHINA | 885 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86 18953170293 | China | 2930 | 58 |