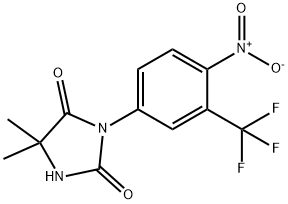
Нилутамид
- английское имяNilutamide
- CAS №63612-50-0
- CBNumberCB3278091
- ФормулаC12H10F3N3O4
- мольный вес317.22
- номер MDLMFCD00864670
- файл Mol63612-50-0.mol
химическое свойство
| Температура плавления | 1490C |
| плотность | 1.463±0.06 g/cm3(Predicted) |
| температура хранения | Sealed in dry,Room Temperature |
| растворимость | Very slightly soluble in water, freely soluble in acetone, soluble in anhydrous ethanol. |
| пка | 7.59±0.70(Predicted) |
| форма | solid |
| цвет | Light yellow to yellow |
| ИнЧИКей | XWXYUMMDTVBTOU-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 63612-50-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 51G6I8B902 |
| Словарь онкологических терминов NCI | Nilandron; nilutamide |
| Словарь наркотиков NCI | Nilandron |
| Код УВД | L02BB02 |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 60-25 | |||||||||
| Заявления о безопасности | 53-36/37/39-45 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | NI9453300 | |||||||||
| кода HS | 2933210000 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
Нилутамид химические свойства, назначение, производство
Описание
Nilutamide is a non-steroidal anti-androgen similar in potency to the acyclic urea flutamide (55) and useful in the management of prostatic carcinoma.Химические свойства
Crystalline SolidИстория
The second nonsteroidal anti-androgen to be marketed was nilutamide. It was discovered from a series of flutamide analogues at Roussel Uclaf (now Sanofi) in the 1970s using a rat prostate assay determining the inhibition of androgen uptake.The investigational compound code was RU 23908. Its structure is closely related to hydroxyflutamide, particularly under the assumption that the α-hydroxyamide engages in an internal hydrogen bond when bound to the AR LBD. The hydantoin moiety of nilutamide mimics the active conformation of hydroxyflutamide.Like flutamide, nilutamide blocks the action of androgens originating from both testis and adrenal. It also has neither agonist nor any other hormonal activity. Nilutamide has an elimination half-life of approximately 2 days in patients, which is significantly longer than that of flutamide. Thus, a single oral dose of 150 mg daily was feasible.
Использование
Nonsteroidal antiandrogen. Antineoplastic (hormonal)Показания
Clinical trials with nilutamide were conducted predominantly in combination with orchiectomy. Results indicated retardation of disease progression and relief of metastatic bone pain in patientswith advanced prostate cancer.However, patient survival benefit was small compared with castration alone. Studies of nilutamide monotherapy or combination with LHRH agonists did not have sufficient patient numbers to allow reliable conclusions on efficacy. Nilutamide was first launched in France in 1987 for treatment of metastatic prostate cancer in adjuvant therapy with surgical castration. Approval in several major markets was granted in the following years.The tolerability profile of nilutamide was similar to that of flutamide. Hot flushes, nausea, diarrhea, constipation, gastrointestinal pain, abnormal liver function, and gynecomastia were frequently reported adverse events with both drugs. Additional side effects were predominantly associated with nilutamide treatment: interstitial pneumonitis, impaired adaptation to darkness, and alcohol intolerance.
The nonsteroidal anti-androgens flutamide and nilutamide established combined androgen blockade as first-line treatment for metastatic prostate cancer. Still, there was room for improvement with regard to overall survival (OS) and tolerability.
Общее описание
Nilutamide, 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione, is usedin combination with surgical castration for the treatment ofmetastatic prostate cancer. Nilutamide, which has an eliminationhalf-life of approximately 40 hours, can also be usedin once-daily dosing, but it has side effects that limit itsuse—visual disturbances, alcohol intolerance, and allergicpneumonitis.Биологическая активность
Non-steroidal and silent antiandrogen. Binds to androgen receptors and also inhibits androgen biosynthesis in vitro . In rats in vivo it inhibits androgen-induced prostate weight increase and inhibits negative androgen-dependent gonadotropin feedback leading to an increase in luteinising hormone and testosterone. Orally active.Механизм действия
The therapeutic effects of nilutamide are overshadowed, however, by the occurrence of several adverse reactions mediated by toxic mechanisms, which are poorly investigated. The reduction of nilutamide is catalyzed by NO synthases via the formation of either or both a nitro anion free radical or its reduction to its hydroxylamino derivative could explain some of the toxic effects of this drug. Nitric oxide synthases also are involved in the formation of reactive NO and oxygen species and in the interactions with some xenobiotic compounds.Клиническое использование
Nilutamide is a hepatotoxic nitroaromatic antiandrogen used for the treatment of metastatic prostate carcinoma in men.Метаболизм
Nilutamide is a nitroaromatic hydantoin analog of flutamide, that is completely absorbed after oral administration, with a mean elimination half-life of approximately 50 hours. One of the methyl groups attached to the hydantoin ring is stereoselectively hydroxylated to a chiral metabolite, which subsequently is oxidized to its carboxylic acid metabolite. Less than 2% of nilutamide is excreted unchanged in the urine. In vitro, the nitro group of nilutamide was reduced to the amine and hydroxylamine moieties by nitric oxide (NO) synthases, a flavin monooxygenase (FMO) system.Нилутамид запасные части и сырье
Нилутамид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 |