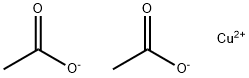
Меди(II) ацета
- английское имяCopper(II) acetate
- CAS №142-71-2
- CBNumberCB2151126
- ФормулаC2H4O2.1/2Cu
- мольный вес181.63
- EINECS205-553-3
- номер MDLMFCD00008690
- файл Mol142-71-2.mol
| Температура плавления | 115°C |
| плотность | 1.92 at 21.9℃ |
| плотность пара | 6.9 (vs air) |
| давление пара | 0.002Pa at 25℃ |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| форма | Powder |
| цвет | Green to blue |
| Flame Color | Green |
| Растворимость в воде | Soluble in water and alcohol. Slightly soluble in ether and glycerol. |
| Чувствительный | Hygroscopic |
| Мерк | 14,2624 |
| БРН | 3595638 |
| Пределы воздействия | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Стабильность | hygroscopic |
| LogP | -0.285 (est) |
| Surface tension | 72mN/m at 1.01-1.08g/L and 21.2-21.6℃ |
| Dissociation constant | 4.69-4.89 |
| Справочник по базе данных CAS | 142-71-2(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | CUPRIC ACETATE |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 39M11XPH03 |
| Система регистрации веществ EPA | Cupric acetate (142-71-2) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | Xn,N | |||||||||
| Заявления о рисках | 22-36/37/38-50/53 | |||||||||
| Заявления о безопасности | 26-60-61-37/39-29 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AG3480000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 9 | |||||||||
| кода HS | 29152990 | |||||||||
| Банк данных об опасных веществах | 142-71-2(Hazardous Substances Data) | |||||||||
| Токсичность | mouse,LD50,intraperitoneal,2500ug/kg (2.5mg/kg),Biochemical Pharmacology. Vol. 30, Pg. 771, 1981. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Меди(II) ацета химические свойства, назначение, производство
Описание
Copper (II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)22 where OAc- is acetate (CH3CO2-). The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu(OAc)2 is a dark green crystalline solid, whereas Cu2(OAc)4(H2O)2 is more bluish-green. Since ancient times, copper acetates of some form have been used as fungicides and green pigments. Today, copper acetates are used as reagents for the synthesis of various inorganic and organic compounds. Copper acetate, like all copper compounds, emits a blue-green glow in a flame.Химические свойства
Cupric acetate is a greenish Blue powder or small crystals.История
Copper (II) acetate was historically prepared in vineyards, since acetic acid is a byproduct of fermentation. Copper sheets were alternately layered with fermented grape skins and dregs left over from wine production and exposed to air. This would leave a blue substance on the outside of the sheet. This was then scraped off and dissolved in water. The resulting solid was used as a pigment, or combined with arsenic trioxide to form copper acetoarsenite, a powerful insecticide and fungicide called Paris Green or Schweinfurt Green.During the Second World War copper acetate was used as shark repellent . Under war conditions, before adoption it has been tested only very briefly (while in general successfully). The source says copper acetate does repel sharks in some situations but not in all.
Использование
Used as a catalyst or oxidizing agent in organic synthesesприкладной
The uses for copper (II) acetate are more plentiful as a catalyst or oxidizing agent in organic syntheses. For example, Cu2(OAc)4 is used to couple two terminal alkynes to make a 1,3-diyne:Cu2(OAc)4 + 2 RC ≡ CH → 2 CuOAc + RC ≡ C-C ≡ CR + 2 HOAc
The reaction proceeds via the intermediacy of copper(I) acetylides, which are then oxidized by the copper(II) acetate, releasing the acetylide radical. A related reaction involving copper acetylides is the synthesis of ynamines, terminal alkynes with amine groups using Cu2(OAc)4.
Общее описание
A blue-green crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Cupric acetate is used as an insecticide, in the preparation of other chemicals, as a fungicide, and mildew preventive.Реакции воздуха и воды
Water soluble.Профиль реактивности
Salts, basic, such as Cupric acetate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Угроза здоровью
Inhalation of dust causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with solutions irritates eyes; contact with solid causes severe eye surface injury and irritation of skin.Пожароопасность
Special Hazards of Combustion Products: Irritating vapors of acetic acid may form in fires.Профиль безопасности
Poison by subcutaneous and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes. See also COPPER COMPOUNDS.Возможный контакт
Cupric acetate is used as a fungicide, as a catalyst for organic reactions; in textile dyeing and as a pigment for ceramics.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
Recystallise it twice from warm dilute acetic acid solutions (5mL/g) by cooling. [Beilstein 2 IV 111.]Несовместимости
Forms explosive materials with acetylene gas, ammonia, caustic solutions; sodium hypobromite; notromethane. Keep away from chemically active metals; strong acids; nitrates. Decomposes above 240C forming acetic acid fumesУтилизация отходов
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill.Меди(II) ацета запасные части и сырье
Меди(II) ацета поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8619931165850 | China | 1000 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +1-858-6993322 | United States | 18526 | 58 |