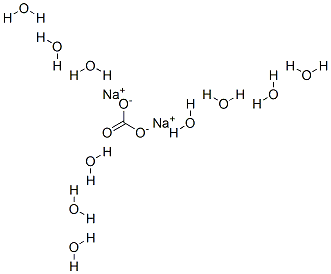
арбонат натрия декагидра
- английское имяSodium carbonate decahydrate
- CAS №6132-02-1
- CBNumberCB8451206
- ФормулаCH20Na2O13
- мольный вес286.14
- EINECS612-116-4
- номер MDLMFCD00149178
- файл Mol6132-02-1.mol
химическое свойство
| Температура плавления | 34 °C |
| плотность | 1.46 |
| Плотность накопления | 700-900kg/m3 |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | H2O: 0.5 M at 20 °C, clear, colorless |
| форма | Small Crystals |
| Удельный вес | 1.46 |
| цвет | White |
| РН | 11-12 (50g/l, H2O, 25℃) |
| Растворимость в воде | 210 g/L (20 ºC) |
| Чувствительный | Air Sensitive |
| Мерк | 14,8596 |
| Справочник по базе данных CAS | 6132-02-1(CAS DataBase Reference) |
| FDA UNII | LS505BG22I |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36 | |||||||||
| Заявления о безопасности | 22-26 | |||||||||
| РИДАДР | 3262 | |||||||||
| WGK Германия | 1 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2836 20 00 | |||||||||
| Группа упаковки | III | |||||||||
| Токсичность | LD50 orally in Rabbit: 4090 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
арбонат натрия декагидра химические свойства, назначение, производство
Химические свойства
Sodium carbonate decahydrate is known as sal soda or washing soda. Its chemical formula is Na2CO3.10H2O. Anhydrous sodium carbonate, Na2CO3, is commonly called soda ash. Sodium carbonate is a white, almost white, or colorless inorganic salt, produced as crystalline powder or granules. It is hygroscopic and odorless with an alkaline taste. The decahydrate, NaCO3.10H2O (washing soda) is a translucent efflorescence crystalline solid. It can be used in the preparation of eutectic hydrate salts, which can be used to remove phase change materials for thermal energy storage. It can also be dehydrated to generate cold heat using a chemical heat pump.Использование
Sodium carbonate decahydrate is used as a raw material in various industries like glass, paper making, soap, detergent, textile and leather. It is also used as a food additive. It is also involved as a precipitation agent of carbonate as well as an analytical reagent in laboratories. It plays an important role as strong base and powerful electrolyte. It finds application in metal refining and cement production.Методы производства
Sodium carbonate is produced by the ammonia-soda process, also known as the Solvay process.Фармацевтические приложения
Sodium carbonate is used as an alkalizing agent in injectable, ophthalmic, oral, and rectal formulations. In effervescent tablets or granules, sodium carbonate is used in combination with an acid, typically citric acid or tartaric acid. When the tablets or granules come into contact with water, an acid– base reaction occurs in which carbon dioxide gas is produced and the product disintegrates. Raw materials with low moisture contents are required to prevent the early triggering of the effervescent reaction.As an alkalizing agent, concentrations of sodium carbonate between 2% and 5% w/w are used in compressed tablet formulations. As an effervescent agent, concentrations of sodium carbonate up to 10% w/w can be used.
Therapeutically, sodium carbonate is also used as an oral antacid.
Безопасность
Sodium carbonate is used in injectable, oral, and rectal pharmaceutical formulations. The pure form of sodium carbonate is mildly toxic by ingestion, moderately toxic by inhalation and SC routes, and very toxic by the IP route. It is irritating to the skin and eyes. Dust and vapors of sodium carbonate may irritate mucous membranes, causing coughing and shortness of breath. It also has experimental reproductive effects.Sodium carbonate can migrate to food from packaging materials. When used as an excipient or antacid, sodium carbonate is generally regarded as a nontoxic and nonirritating material.
LD50 (mouse, IP): 0.12 g/kg
LD50 (mouse, SC): 2.21 g/kg
LD50 (rat, oral): 4.09 g/kg
хранилище
Sodium carbonate converts to the monohydrate form when in contact with water and produces heat. It begins to lose carbon dioxide at temperatures above 400℃ and decomposes before boiling. Store in airtight containers.Несовместимости
Sodium carbonate decomposes when in contact with acids in the presence of water to produce carbon dioxide and effervescence. It may react violently with aluminum, phosphorous pentoxide, sulfuric acid, fluorine, and lithium.Регуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections; ophthalmic solution; oral capsules and tablets; rectal suspensions). Included in the Canadian List of Acceptable Non-medicinal Ingredients. Included in parenteral (powder for solution for injection) and nonparenteral medicines (oral effervescent tablets, soluble tablets, granules, lozenges, chewing gums) licensed in the UK.USP32–NF27 allows either the anhydrous or the monohydrate form.
арбонат натрия декагидра запасные части и сырье
запасной предмет
арбонат натрия декагидра поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 15369953316 +8615369953316 |
China | 2123 | 58 | |
| +8615255079626 | China | 23541 | 58 |
арбонат натрия декагидра Обзор)
1of4