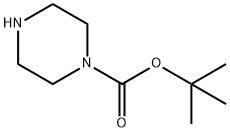
N-Boc-piperazine is a compound useful in organic synthesis.
1-Boc-piperazine may be used in:
- preparation of series of (m-phenoxy)phenyl substituted piperazine derivatives
- termination step during synthesis of α,β-poly(2-oxazoline) lipopolymers via living cationic ring opening polymerization
- preparation of monosubstituted piperazines, e.g. in the synthesis of indazole DNA gyrase inhibitors
1-Boc-piperazine undergoes Buchwald-Hartwig amination with various aryl halides to form corresponding amine derivatives. It can be used to synthesize monosubstituted piperazine intermediates of many bioactive molecules and piperazine containing drug substances, such as trazodone.
Tert-Butyl 1-piperazinecarboxylate, also known as Olaparib Impurity 7, is an impurity of Olaparib. Olaparib is used alone or in combination with bevacizumab (Avastin) to help maintain the response of certain types of ovarian (female reproductive organs where eggs are formed), fallopian tube (tube that transports eggs released by the ovaries to the uterus), and peritoneal (layer of tissue that lines the abdomen) cancer in people who have completely responded or partially responded to their first or later chemotherapy treatments. Olaparib is also used to treat certain types of breast cancer that has spread to other parts of the body and has not improved or has worsened after treatment with other therapies. It is also used to treat certain types of early breast cancer in people who have already been treated with other chemotherapy treatments.
1-Boc-piperazine is an N-Boc protected piperazine. Crosscoupling of 1-Boc-piperazine with aryl iodides using CuBr/1,1′-bi-2-naphthol (catalyst) and K3PO4 (base) has been reported. 1-Boc-piperazine undergoes Buchwald-Hartwig coupling reactions with aryl halides. 1-Boc-piperazine can be prepared in 80% yield via solvent-free N-Boc protection catalyzed by iodine.
The current method of synthesizing 1-tert-butoxycarbonylpiperazine is mainly to use anhydrous piperazine drops with di-tert-butyl dicarbonate selective reaction of the nitrogen at one end of the piperazine is protected so that the other end of the amino group to go to the reaction with the other group, so that the method of reaction, the piperazine will be connected to the ends of the di-tert-butyl dicarbonate, the purification of the purity of the piperazine must be washed with a large amount of water.