Different applications of 1-tert-Butoxycarbonylpiperazine
Introdction
1-tert-Butoxycarbonylpiperazine is an important chemical product. 1-tert-Butoxycarbonylpiperazine can be used as a raw material to synthesize various chemical and medical products. 1-tert-Butoxycarbonylpiperazine is widely used in the chemical industry. Tert-butyl 1-piperazinecarboxylate was employed to react with p-Toluoyl chloride in basic condition to provide compound.

Picture 1 1-tert-Butoxycarbonylpiperazine powders
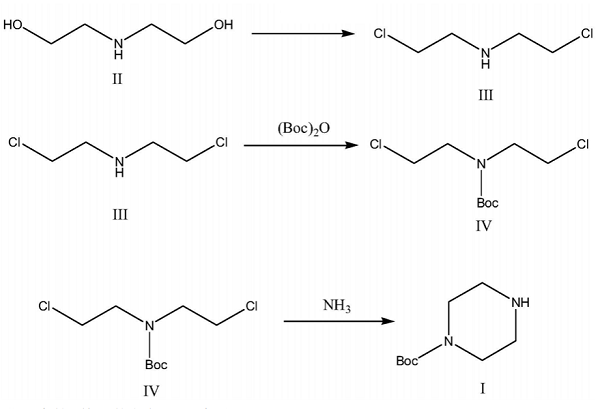
At present, the method of synthesizing 1-tert-butoxycarbonylpiperazine is mainly to use anhydrous piperazine to add di-tert-butyl dicarbonate to selectively react to protect the nitrogen at one end of the piperazine, so that the amino group at the other end is removed from another group. Reaction, such a reaction method, both ends of piperazine will be connected with di-tert-butyl dicarbonate, and must be washed with a large amount of water during purification.
Application in preparation of tert-butyl 1-piperazine carboxylate 6
A solution of di-tert-butyl dicarbonate (2.93 g, 12.77mmol) in MeOH (25mL) was slowly added to a stirring solution of piperazine (2.00 g, 23.22mmol) in MeOH (50mL) at 0 C. The mixture was then stirred for 2 days at room temperature, and the solvent removed in vacuum. The crude solid was redissolved in diethyl ether (100mL) with warming, and the white precipitate left was filtered off. The product was extracted from the mother liquor with 1M citric acid solution (3 · 50mL), and the aqueous layer was washed with Et2OAc (3 · 50mL), basified with Na2CO3 (pH 11), and extracted with Et2OAc (3 · 50mL). The organic layer was dried over Na2SO4 and evaporated in vacuum to give tert-butyl 1-piperazinecarboxylate 6 as a waxy white solid (crude, 1.57 g, 66%), mp = 53–54C. 1 H NMR (250MHz, CDCl3) 1.42 (s, 9H), 1.89 (s, 1NH), 2.78 (m, 4H), 3.36 (m, 4H)[1].
Application in preparation of tert-butyl 4-propargylpiperazine-1-carboxylate
Propargyl bromide (356.9mg, 3mmol) was added slowly to a mixture of tert-butyl 1-piperazinecarboxylate 6. (558.8mg, 3mmol) and diisopropylethylamine (407.1mg, 3.15mmol) in CHCl3 (25mL) at 0 C. The mixture was stirred for 24 h at room temperature. CHCl3 (50mL) was then added and the solution obtained was washed with 5% NaHCO3 (3 · 50mL), brine (2 · 50mL), and then dried over Na2SO4. The solution was filtered and evaporated to dryness. The residue was crystallized from a mixture of benzene–hexane (1:1) and gave tert-butyl 4-propargylpiperazine-1-carboxylate 7 (337mg, 86%). 1 H NMR (250MHz, CDCl3): 1.42 (s, 9H), 2.22 (s, 1H), 2.46 (s, 4H), 3.26 (s, 2H), 3.41 (s, 4H)[2].
Application in preparation of tert-Butyl 4-(4-nitrobenzyl)-1-piperazinecarboxylate
To a solution of tert-butyl 1-piperazinecarboxylate, 1 (0.74 g, 4 mmol), and 4-nitrobenzyl bromide, 2 (0.86 g, 4 mmol), in N,N-dimethylformamide (DMF, 5 mL) was added potassium carbonate (3 g), and the reaction mixture was stirred at room temperature for 4 h. The product was extracted with ethyl acetate (50 mL), and the organic phase was washed with water (20 mL · 3). The solution was dried with sodium sulfate and the solvent was removed in vacuo. The product was purified by column chromatography, eluting with ethyl acetate to afford as a solid (1.22 g, 95% yield), mp 99–100 C. 1 H NMR (DMSO-d6): 8.21–8.19 (d, 2H, J = 8.8 Hz, ArH), 7.61–7.59 (d, 2H, J = 8.0 Hz, ArH), 3.63 (s, 2H, CH2), 3.34–3.30 (m, 4H, partially obscured, 2· CH2), 2.35–2.32 (m, 4H, 2· CH2), 1.39 (s, 9H, 3· CH3). Anal. (C16H23N3O4) C, H, N[3].
Application in preparation of tert-Butyl 4-(4-nitrobenzyl)-1-piperazinecarboxylate
(6-Chloronaphthalen-2-yl)sulfonylchloride was synthesized via b-sulfonylation and successive chlorination of 2-chloronaphthalene. Compound 8 was treated with tert-butyl 1-piperazinecarboxylate and the resulting compound was deprotected to give 1-(6-chloronaph[1]thalene-2-sulfonyl)piperazine. On the other hand, compound 8 was treated with 2-ethoxy-carbonylpiperazine to obtain 1-Boc-4-(6-chloronaphthalen-2-yl)sulfonylpiperazine-2-carboxylic acid. Compound 9b was converted to 4-(6-chloronaphthalen-2-yl)sulfonyl-2-carbamoylpiperazines 9c–i by reactions with a series of amines.
Reference
1 Zheng, Hailin, et al. "Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases." Bioorganic & medicinal chemistry 13.3 (2005): 773-783.
2 Rice, Kenneth D., et al. "Dibasic inhibitors of human mast cell tryptase. Part 1: synthesis and optimization of a novel class of inhibitors." Bioorganic & medicinal chemistry letters 10.20 (2000): 2357-2360.
3 Wang, Yuqiang, et al. "Synthesis and evaluation of a DHA and 10-hydroxycamptothecin conjugate." Bioorganic & medicinal chemistry 13.19 (2005): 5592-5599.
You may like
Related articles And Qustion
See also
Lastest Price from 1-BOC-Piperazine manufacturers

US $0.00/kg2025-07-05
- CAS:
- 57260-71-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $19.90/kg2025-04-21
- CAS:
- 57260-71-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt