Application of 3-O-ethyl-L-ascorbic acid
Introduction
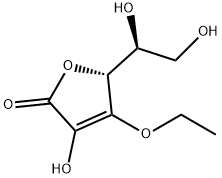
AA is a dibasic acidic unsaturated lactone, which con[1]sists of an electron rich C2 - C3 enediol moiety in a five-membered ring. This structure predisposes the molecule to oxidation and instability under either aerobic or anaerobic conditions. L-ascorbic acid (AA), commonly known as vitamin C, has been widely used in topical formulations for many years as an antioxidant and anti-aging ingredient. However, the physicochemical properties of AA are not optimal for skin uptake and the molecule is also unstable, readily undergoing oxidation on exposure to air. The compound 3-o-ethyl-l-ascorbic acid (EA) has been developed as a stable vitamin C derivative and has been used in topical products[1].

Picture 1 3-O-Ethyl-L-ascorbic powders
EA has been used in cosmetic products as a stable alternative to AA for a number of years. Additionally, EA containing products have been used for skin whitening and topical EA has been reported to be effective in treating medical conditions of hyper-pigmentation, such as melasma. Finally, because of its reducing ability, EA should be investigated as an antioxidant ingredient to increase stability of pharmaceutical formulations[2].
Properties
3-O-ethyl-l-ascorbic acid (EA) is an l-ascorbic acid derivative with an ethyl group at the third carbon position. This structural modification protects the 3-OH group from ionization, and thus the molecule from oxidation, but also results in changes in the physicochemical properties. With regards to EA safety, to date, only two cases of allergic contact dermatitis have been reported in the literature. The first case was reported in 2014 (Yagami et al.), when a female patient developed an itchy erythematous rash after applying a 2% EA lotion to the face. Patch testing with the ingredients of the formulation indicated that EA was the causative allergen and showed the minimum positive concentration to be 0.05% EA. One year later, a second case of allergic contact dermatitis due to EA were reported. A 51-year female was found to have an allergic reaction to EA after patch testing, with a minimum positive concentration of 1% EA[1].
Application in topical products
Even though EA is a compound widely used in topical products (Jeon et al., 2016; Silva et al., 2019), surprisingly, its physicochemical properties have not been reported to date. Additionally, the skin permeation profile of EA has not been investigated nor have the effects of different vehicles on skin uptake of EA been examined. The objectives of the present work were therefore (i) to undertake a comprehensive characterisation of EA, (ii) to assess its solubility and stability in a range of solvents commonly used in topical preparations and (iii) to investigate skin delivery of EA from these solvents[1].
Stability of EA in the solvents
Stability of EA in the solvents was investigated for 120 h (5 d) at 32 ± 1 °C. Solutions of a known concentration of EA were prepared in triplicate and placed in an Eppendorf ® tube. The sample was sealed with Parafilm® (Bemis Inc., U.S.A.) and placed in a shaking incubator, as for solubility studies. Samples were collected at 0, 24, 48, 72, 96 and 120 h. All samples were subsequently diluted and analysed by HPLC[2].
The saturation solubility values
The saturation solubility values of EA in the solvents studied are shown. The calculated solubility parameters of vehicles are also shown. The solubility parameter of EA was calculated as 15.4 ((cal/cm3 ) 1/2). EA solubility ranged from 463.6 ± 1.3 mg/ml to 801.1 ± 1.7 mg/ml for the hydrophilic solvents. Lower solubility was found for the lipophilic solvents Lab, IPM, PGML and PGMC, ranging from 0.6 ± 0.2 mg/ml to 56.6 ± 1.3 mg/ml. Of all the vehicles ex[1]amined, EA was found to be less stable in TriPG and DiPG after 120 h, with recovery values of 76.64 and 82.76% respectively. In all other solvents, recovery of EA ranged from 90.23% to 101.51%[2].
Reference
1 Iliopoulos F, Sil B C, Moore D J, et al. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin[J]. International journal of pharmaceutics: X, 2019, 1: 100025.
2 Jin S, Miao X. 3-O-Ethyl-L-ascorbic acid[J]. Acta Crystallographica Section E: Structure Reports Online, 2008, 64(5): o860-o860.
Related articles And Qustion
See also
Lastest Price from 3-O-Ethyl-L-ascorbic acid manufacturers

US $0.00/kg2025-11-21
- CAS:
- 86404-04-8
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 1000 kg

US $0.00/kg2025-09-17
- CAS:
- 86404-04-8
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 1kg/5kg25kg