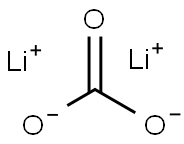
Different applications of lithium carbonate
Introduction
Lithium carbonate(Li2CO3) is an inorganic compound. It could be slightly soluble in water and dilute acid, insoluble in ethanol and acetone. The thermal stability is lower than carbonates of other elements of the same family in the periodic table, and it does not deliquesce in the air. It can be obtained by adding sodium carbonate to lithium sulfate or lithium oxide solution. It can be converted into acid salt by introducing carbon dioxide into its aqueous solution, which can be hydrolyzed by boiling. It is used as raw materials for ceramics, glass, ferrite, etc., components sprayed with silver paste, etc., and is used in medicine to treat mental depression [1].

Picture 1 Lithium carbonate powders
The application in mania
Lithium carbonate has been widely acclaimed as a useful drug in the treatment of manic-depressive psychoses. The results of this clinical study emphasize that lithium is highly beneficial in preventing and alleviating acute mania and the hypomanic aspects of schizo-affective psychoses. When used alone, lithium was found to be relatively ineffective as an antidepressant. However, in combination with a tricyclic or MAO-inhibiting antidepressant, it was often effective in alleviating depressions, including those refractory to single psychopharmacological agents.
Lithium carbonate is probably the most effective therapy available for mania. It can produce euthymia without sedation. Lithium carbonate treatment of the acute manic state, however, requires the use of large but subtoxic dosages. The time required for its effect is different from that of other neuroleptics; usually at least five to ten days pass before full benefits are achieved.6 ' In order to overcome the disadvantage of delayed effect, a combined regimen of lithium carbonate and haloperidol at varying dosages has been advocated. The purpose of the high doses of haloperidol is to get immediate amelioration of mania while awaiting the later, more specific effect of lithium carbon¬ ate, which can then be used as the maintenance drug. This report concerns four acutely agitated patients with the diagnosis of mania who were treated with a combined regimen of lithium carbonate (Lithane) and high doses of haloperidol (Haldol) in the Psychiatry Service of the Metropolitan Hospital. At least 50 other patients had been similarly treated without reported adverse effects. In these four, severe encephalopathic syndromes developed. Two of the patients suffered widespread, irreversible brain damage and now exhibit gross neurologic deficits, which, to our knowledge, have never been seen after treatment with these drugs. The other patients emerged from their acute toxic reaction with persistent dyskinesias.
The application in battery chemistries
Lithium carbonate (1873) n. Li2CO3. A crystalline salt used in the glass and ceramic industries. because of its highest theoretical specific energy among all known battery chemistries. However, parasitic reactions bring about vexing issues on the efficiency and longevity of LAB, among which the formation and decomposition of lithium carbonate, Li2CO3, is of paramount importance. The discovery of Li2CO3 as the main discharge product in carbonate-based electrolytes once brought researchers to ‘the end of the idyll’ in the early 2010s. In the past few years, tremendous efforts have been made to understand the formation and decomposition mechanisms of Li2CO3, as well as to conceive novel chemical/material strategies to suppress the Li2CO3 formation and to facilitate the Li2CO3 decomposition. Moreover, the study on Li2CO3 in LABs is opening up a new research field of the Li − CO2 battery. Considering the rapid development and innumerous emerging issues, it is timely to recapitulate the current understandings, define the ambiguities and scientific gaps, and discuss the topics of high priority for future research, which are the aims of this Minireview.
The formation of Li2CO3
In this section, we discuss the formation of Li2CO3 on the cathode side. There are mainly three sources: decomposition of the electrolyte, chemical reactions between the carbon electrode and Li2O2/LiO2, and parasitic reactions involving residual CO2 in ambient air, as summarized in Figure 2. It should be noted that the formation of Li2CO3 on the anode side due to the reaction between the electrolyte and the Li anode will nnot be discussed, as it is often associated with the topic of solid electrolyte interface (SEI) film, which has been extensively reviewed recently.[10] Figure 2. Formation mechanisms of Li2CO3 in LABs, which are classified into three sources: (i) electrolyte decomposition, (ii) reaction between the carbon electrode and Li2O2/LiO2 and (iii) reaction involving CO2. Different reaction pathways, different morphologies and chemical compositions of the discharge products depending on the electrolyte properties are discerned in the former two sources. It is widely acknowledged that film-like Li2O2 is formed in low DN electrolytes and toroidal Li2O2 is formed in high donor number (DN) electrolytes.
Reference
1 Zall H, THERMAN P E R O G, Myers J M. Lithium carbonate: a clinical study[J]. American Journal of Psychiatry, 1968, 125(4): 549-555.
2 Cohen W J, Cohen N H. Lithium carbonate, haloperidol, and irreversible brain damage[J]. Jama, 1974, 230(9): 1283-1287.
3 Zhao Z, Huang J, Peng Z. Achilles’ Heel of lithium–air batteries: Lithium carbonate[J]. Angewandte Chemie International Edition, 2018, 57(15): 3874-3886.
4 Sarantidis D, Waters B. A review and controlled study of cutaneous conditions associated with lithium carbonate[J]. The British Journal of Psychiatry, 1983, 143(1): 42-50.
Related articles And Qustion
See also
Lastest Price from Lithium carbonate manufacturers

US $1.00-4.00/KG2025-09-12
- CAS:
- 554-13-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $1.00-4.00/KG2025-09-12
- CAS:
- 554-13-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG