Lithium Carbonate: A Critical Compound in Modern Chemistry
Introduction
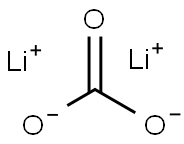
Lithium carbonate, with the chemical formula Li₂CO₃, is an inorganic compound of considerable importance in various industries, particularly in the fields of medicine and energy storage. It is a white, odorless, crystalline powder that is highly valued for its role in the production of lithium-ion batteries, which power a wide array of electronic devices, from smartphones to electric vehicles.

Figure 1 Characteristics of Lithium carbonate
Applications
Affective Disorders1
A clinical study of lithium carbonate in affective disorders was undertaken by Arry Zall and colleagues. The resuits of this study indicated the following:
1. Lithium brought about a complete recovery in most of the acutely ill manic patients and a partial remission in the others.
2. Lithium used alone was relatively ineffective in preventing and relieving acute depressions. Lithium in combination with tricyclic antidepressants and MAO inhibitors was more effective.
3. Lithium usually corrects the affective component of a schizo-affective psychosis, but does not alter the accompanying thought disorder.
4. Lithium carbonate can be used safely in out-patient therapy for maintenance or acute treatment if laboratory and clinical follow-ups are scheduled regularly.
5. Toxicity from lithium when it is given to medicallytients in recommended doses.
Mania2
Mania is taken to mean here an excitability of mood and movement associated with rapid, forceful thinking, in the absence of evidence of incongruity of affect. All patients had recently developed the acute phase of the illness, the peak of which lay within a period of three months from the time investigations had commenced. From the very nature of the manic-depressive psychosis it is clear that some patients will have had no previous attack of mania or depression, whilst others may have had many.
Of the 28 patients (11 male and 17 female), who were accepted for the trial and lithium carbonate treatment, nine did not complete the whole cycle of six weeks' treatment for the following reasons: two showed toxic effects, two left hospital against medical advice, and five be came so disturbed in behaviour as to be beyond management. The results from 18 patients only have been used in the statistical assessment. For reasons given later the Wittenborn Scale was used to assess clinical changes.
Result shows that the degree of mania diminished significantly during the second week of treatment with lithium carbonate compared with the effect of placebo at a similar time. An analysis of variance based upon a square root trans formation of the figures was made, and using a two-tailed "t" test the difference between means at the end of two weeks' treatment was significant at the 0.05 level (Cluster III) or 0.02 level (Cluster V). These differences were significant whether placebo or lithium carbonate were administered first. An analysis of the figures at the end of the first week showed a less marked treatment effect and it was only in Group B that the difference in the means was significant at the 0.05 level on both Clusters III and V using a one-tailed "t" test.
References:
[1] FORDHAM M. A clinical study[C]. 2018. DOI:10.4324/9780429474422-11.[2] MAGGS R. Treatment of Manic Illness with Lithium Carbonate[J]. British Journal of Psychiatry, 1963, 109 1: 56-65. DOI:10.1192/bjp.109.458.56.
You may like
Related articles And Qustion
See also
Lastest Price from Lithium carbonate manufacturers

US $1.00-4.00/KG2025-09-12
- CAS:
- 554-13-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $1.00-4.00/KG2025-09-12
- CAS:
- 554-13-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG