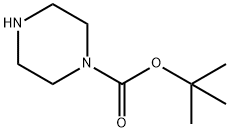
The synthesis method of 1-BOC-Piperazine
Introduction
N-Boc piperazine (1-BOC-Piperazine) belongs to the monosubstituted piperazine compounds. 1-Boc-piperazine reacts with various aromatic halides to form corresponding amine derivatives by Buchwald-Hartwig amination. Due to their special structure, monosubstituted piperazine compounds have become key intermediates in synthesising many drugs, such as glucocorticoids such as palbociclib, ranolazine, and dexamethasone. Therefore, synthesising monosubstituted piperazine compounds has very important pharmaceutical and economic value.
Synthesis method
At present, there are two main methods for synthesizing 1-BOC-Piperazine:
Method 1: Use anhydrous piperazine to add di-tert-butyl dicarbonate to selectively react, protect the nitrogen at one end of piperazine, and allow the amino group at the other end to react with other groups. In the above method, both ends of piperazine will be connected to di-tert-butyl dicarbonate, and a large amount of water must be used for washing during purification, which reduces the yield of the product; it also increases the cost of three waste treatments, and the cost of raw materials for this process is high.
Method 2: Starting from piperazine, it reacts with glacial acetic acid to form a salt, then di-tert-butyl dicarbonate is added for acylation reaction, and finally the crude N-tert-butyloxycarbonyl piperazine is extracted by toluene; in the post-treatment, ethyl acetate is used as an extractant to remove unreacted raw materials and other impurities, and finally a qualified 1-BOC-Piperazine product is obtained. Although the above method improves the selectivity of N on piperazine, it uses anhydrous piperazine as the main raw material, the cost is relatively high, and toluene is used, which poses a threat to the health of the staff.
Based on the shortcomings of the above synthesis method, Liang et al. overcame the shortcomings of the above prior art and provided a method for synthesizing 1-BOC-Piperazine. The method uses diethanolamine as the starting material and synthesizes N-Boc piperazine in three steps chlorination, Boc protection, and ammonolysis and cyclization. The raw materials of this method are easy to obtain, the reaction conditions are mild, the product cost is low, the yield is high, the purity is high, and it is suitable for industrial production.
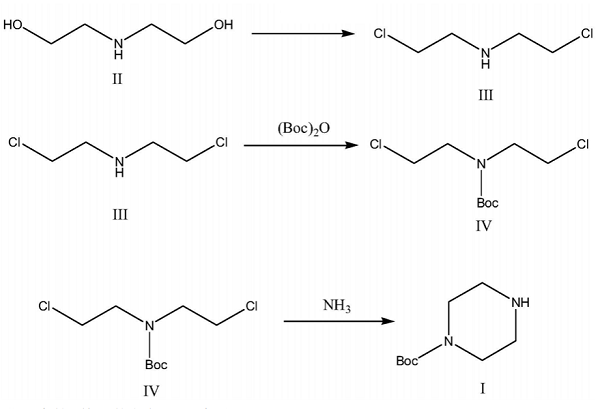
Specific synthesis steps:
1) Add 327g (2.75mol) thionyl chloride to a 2000ml reaction bottle, drop 105g (1mol) diethanolamine, heat and reflux for 4.5h; cool to below 10℃, add 1000ml purified water and stir until no heat is released;
2) Add 583g (5.5mol) sodium carbonate to dissolve, pH>10; control the temperature within 10-20℃, drop 214g (0.98mol) Boc anhydride, react at 25℃ for 14 hours, keep the reaction system alkaline;
3) Heat to 60℃, slowly drop 170g (2.8mol) ammonia water, and drip for about 3h; control the temperature at 60℃ to react for 3.5h, cool below 25℃, extract the reaction solution with 900ml ethyl acetate three times, dry the ethyl acetate layer with sodium sulfate, filter, evaporate the ethyl acetate under reduced pressure below 60℃, and cool to obtain 1-BOC-Piperazine 174 .5g, yield 93.8%, purity 99.72%.
References
[1] CN108033931B.pdf (storage.googleapis.com) https://patentimages.storage.googleapis.com/5e/02/24/e6f37d9c560e9d/CN108033931B.pdf
You may like
Related articles And Qustion
Lastest Price from 1-BOC-Piperazine manufacturers

US $0.00/kg2025-07-05
- CAS:
- 57260-71-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $19.90/kg2025-04-21
- CAS:
- 57260-71-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt