Glutamic acid derivatives
Glutamic acid,Scientific name: α- aminoglutaric acid —so named because it is extracted from grains. α- aminoglutaric acid is widespread and almost can be found in all proteins. Glutamic acid is a protein hydrolysate, a colorless massive crystalline. It is soluble in water while insoluble in benzene, phenol and other organic solvent. Glutamic acid is an acidic amino acid because its aqueous solution is acidic. Under the physiological condition, glutamic acid unites with glycine and cysteine to form glutathione, a substance with important physiological function. Glutamic acid possesses umami, and its sodium salt is monosodium glutamate (MSG). MSG will decompose at high temperatures, and is vulnerable to great heat.
MSG is first discovered by Japanese chemist Ikeda. He discovered it by chance when he found the kelp soup containing a delicate flavour. On investigation, he has extracted 2g MSG from 100 kg kelp. A large number of MSG which called Ajinomoto have produced in Japan later. In 1930s, the Ajinomoto in China has produced by Shanghai TianChu Ajinomoto Factory. These products are exported to countries and receive the prestige. Hydrolyzing with natural gliadin or casein, adjusting pH to 3.3 to obtain glutamic acid precipitation, adding an appropriate amount of sodium hydroxide after separating can get MSG. MSG can also be synthesized but the cost is expensive, and they are usually still made by protein hydrolysis. After 1960s, Japanese use microbial fermentation to produce of glutamic acid. They put Curtobacterium, Chlorella, Bacillus into carbohydrate (mainly starch and glucose). Then they add urea, ammonium sulfate, ammonium chloride and an appropriate amount of biotin. These bacteria secrete large amounts of glutamic acid immediately to produce MSG. At present, China takes the method of fermentation to produce MSG. Glutamate can also combine with ammonia in the blood to form a nontoxic substance. So glutamate can also be used to treat diseases like hepatic coma and liver dysfunction during convalescence.
- Structure:
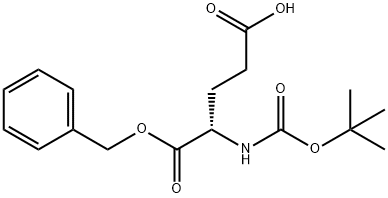
- Chemical Name:Boc-L-Glutamic acid 1-benzyl ester
- CAS:30924-93-7
- MF:C17H23NO6
- Structure:
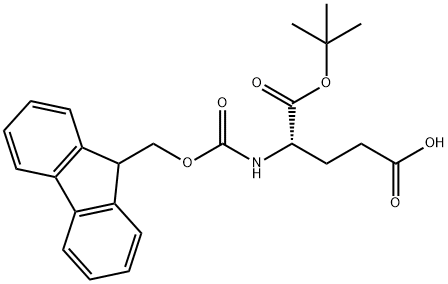
- Chemical Name:Fmoc-L-Glutamic acid 1-tert-butyl ester
- CAS:84793-07-7
- MF:C24H27NO6
- Structure:
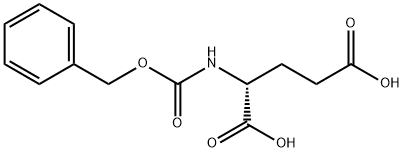
- Chemical Name:Z-D-GLU-OH
- CAS:63648-73-7
- MF:C13H15NO6
- Structure:
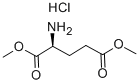
- Chemical Name:L-Glutamic acid dimethyl ester hydrochloride
- CAS:23150-65-4
- MF:C7H14ClNO4
- Structure:
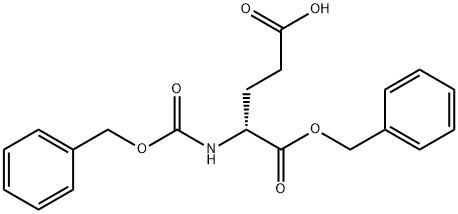
- Chemical Name:N-Cbz-D-glutamic acid alpha-benzyl ester
- CAS:65706-99-2
- MF:C20H21NO6
- Structure:
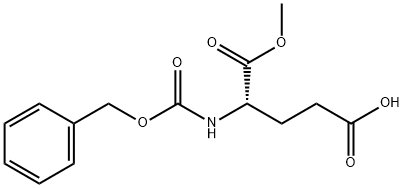
- Chemical Name:Z-GLU-OME
- CAS:5672-83-3
- MF:C14H17NO6
- Structure:
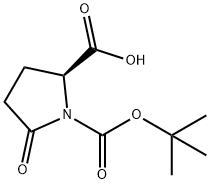
- Chemical Name:BOC-PYR-OH
- CAS:53100-44-0
- MF:C10H15NO5
- Structure:
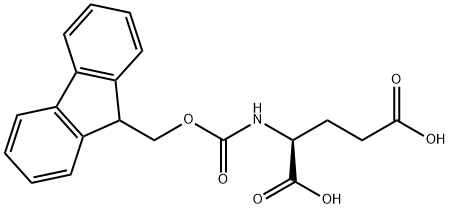
- Chemical Name:Fmoc-L-glutamic acid
- CAS:121343-82-6
- MF:C20H19NO6
- Structure:
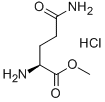
- Chemical Name:L-GLUTAMINE METHYL ESTER HYDROCHLORIDE
- CAS:32668-14-7
- MF:C6H13ClN2O3
- Structure:
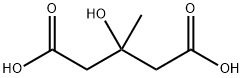
- Chemical Name:3-HYDROXY-3-METHYLGLUTARIC ACID
- CAS:503-49-1
- MF:C6H10O5
- Structure:
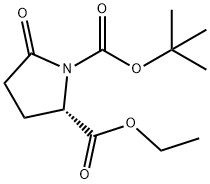
- Chemical Name:BOC-PYR-OET
- CAS:144978-12-1
- MF:C12H19NO5
- Structure:
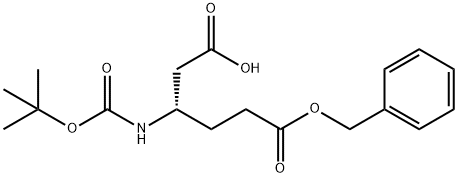
- Chemical Name:Boc-L-beta-homoglutamic acid 6-benzyl ester
- CAS:218943-30-7
- MF:C18H25NO6
- Structure:
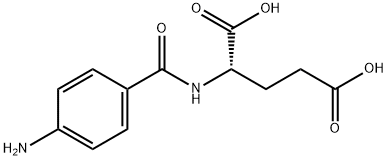
- Chemical Name:N-(p-Aminobenzoyl)glutamic acid
- CAS:4271-30-1
- MF:C12H14N2O5
- Structure:
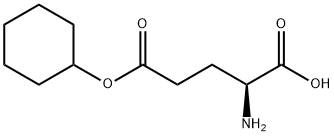
- Chemical Name:L-Glutamic acid 5-cyclohexyl ester
- CAS:112471-82-6
- MF:C11H19NO4
- Structure:
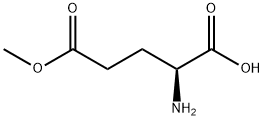
- Chemical Name:L-Glutamic acid 5-methyl ester
- CAS:1499-55-4
- MF:C6H11NO4
- Structure:
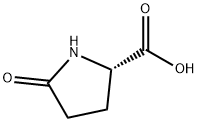
- Chemical Name:DL-Pyroglutamic acid
- CAS:149-87-1
- MF:C5H7NO3
- Structure:
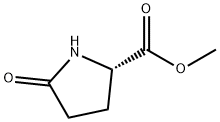
- Chemical Name:Methyl L-pyroglutamate
- CAS:4931-66-2
- MF:C6H9NO3
- Structure:
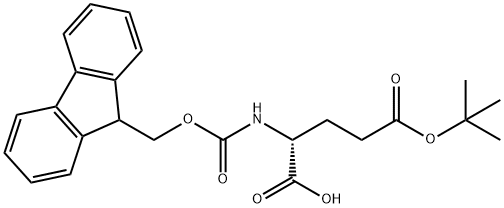
- Chemical Name:Fmoc-D-glutamic acid gamma-tert-butyl ester
- CAS:104091-08-9
- MF:C24H27NO6
- Structure:
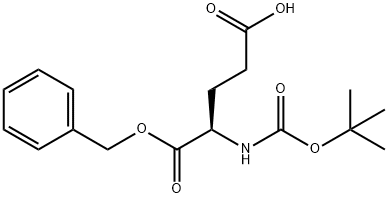
- Chemical Name:Boc-D-Glu-OBzl
- CAS:34404-30-3
- MF:C17H23NO6
- Structure:
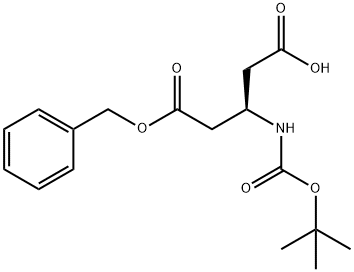
- Chemical Name:Boc-L-beta-glutamic acid 5-benzyl ester
- CAS:254101-10-5
- MF:C17H23NO6
- Structure:
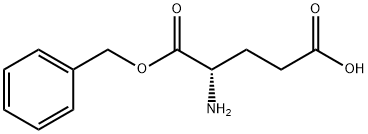
- Chemical Name:L-Glutamic acid alpha-benzyl ester
- CAS:13030-09-6
- MF:C12H15NO4
- Structure:
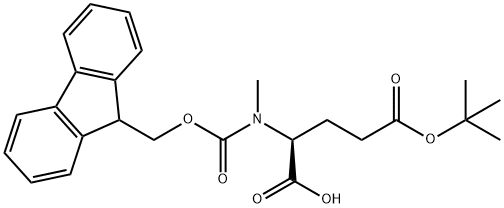
- Chemical Name:Fmoc-N-methyl-L-glutamic acid 5-tert-butyl ester
- CAS:200616-40-6
- MF:C25H29NO6
- Structure:
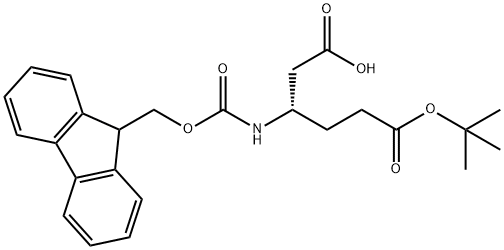
- Chemical Name:Fmoc-L-beta-homoglutamic acid 6-tert-butyl ester
- CAS:203854-49-3
- MF:C25H29NO6
- Structure:
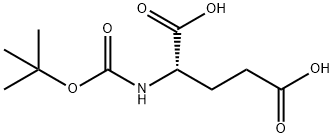
- Chemical Name:Boc-L-Glutamic acid
- CAS:2419-94-5
- MF:C10H17NO6
- Structure:
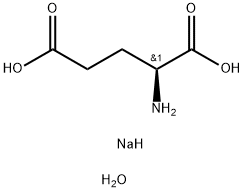
- Chemical Name:L(+)-Monosodium glutamate monohydrate
- CAS:6106-04-3
- MF:C5H12NNaO5
- Structure:
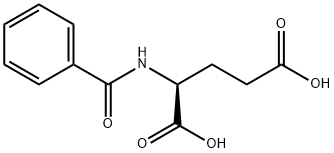
- Chemical Name:BZ-GLU-OH
- CAS:6094-36-6
- MF:C12H13NO5
- Structure:
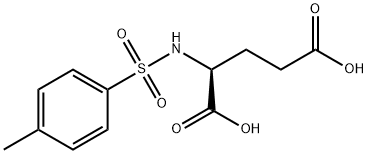
- Chemical Name:N-(p-Tolylsulphonyl)-L-glutamic acid
- CAS:4816-80-2
- MF:C12H15NO6S
- Structure:
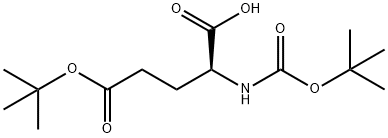
- Chemical Name:N-tert-Butoxycarbonyl-L-glutamic acid gamma-tert-butyl ester
- CAS:13726-84-6
- MF:C14H25NO6
- Structure:
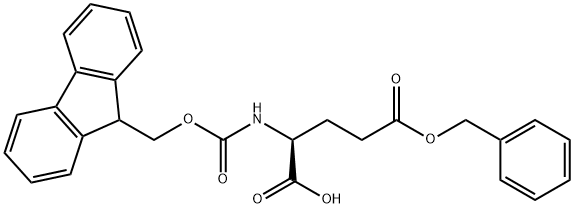
- Chemical Name:Fmoc-L-glutamic acid-gamma-benzyl ester
- CAS:123639-61-2
- MF:C27H25NO6
- Structure:
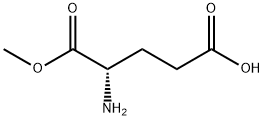
- Chemical Name:H-Glu-OMe
- CAS:6384-08-3
- MF:C6H11NO4
- Structure:
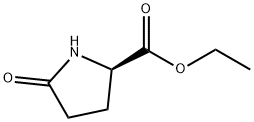
- Chemical Name:Ethyl D-(-)-pyroglutamate
- CAS:68766-96-1
- MF:C7H11NO3
- Structure:
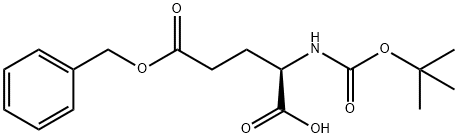
- Chemical Name:Boc-D-Glutamic acid 5-benzyl ester
- CAS:35793-73-8
- MF:C17H23NO6
- Structure:
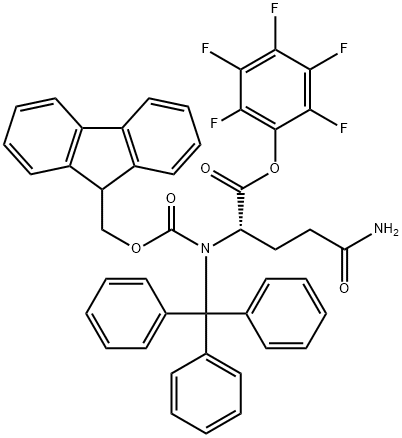
- Chemical Name:FMOC-GLN(TRT)-OPFP
- CAS:132388-65-9
- MF:C45H33F5N2O5
- Structure:
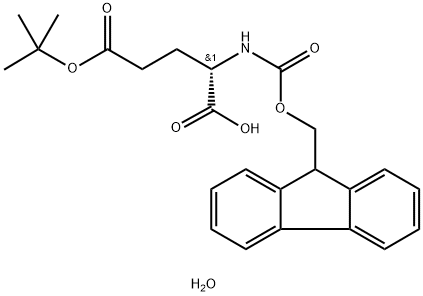
- Chemical Name:FMOC-GLU(OTBU)-OH H2O
- CAS:204251-24-1
- MF:C24H29NO7
- Structure:
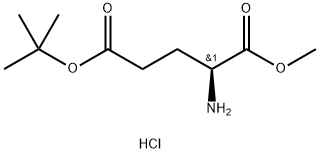
- Chemical Name:L-Glutamic acid 5-tert-butyl 1-methyl ester hydrochloride
- CAS:6234-01-1
- MF:C10H20ClNO4
- Structure:
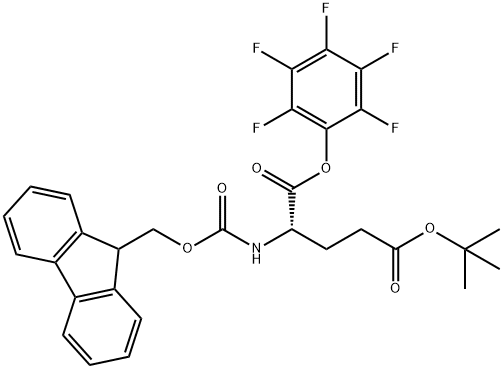
- Chemical Name:FMOC-GLU(OTBU)-OPFP
- CAS:86061-04-3
- MF:C30H26F5NO6
- Structure:
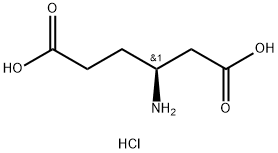
- Chemical Name:L-beta-Homoglutamic acid hydrochloride
- CAS:61884-74-0
- MF:C6H12ClNO4
- Structure:
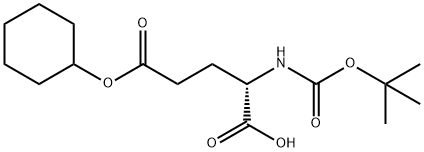
- Chemical Name:Boc-L-glutamic acid 5-cyclohexyl ester
- CAS:73821-97-3
- MF:C16H27NO6
- Structure:
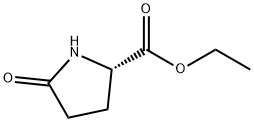
- Chemical Name:Ethyl L-pyroglutamate
- CAS:7149-65-7
- MF:C7H11NO3
- Structure:
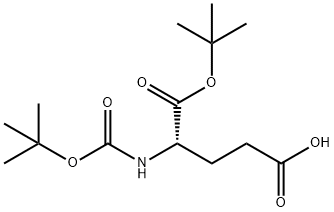
- Chemical Name:Boc-L-glutamic acid 1-tert-butyl ester
- CAS:24277-39-2
- MF:C14H25NO6
- Structure:
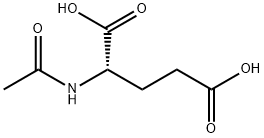
- Chemical Name:N-Acetyl-DL-glutamic acid
- CAS:5817-08-3
- MF:C7H11NO5
- Structure:
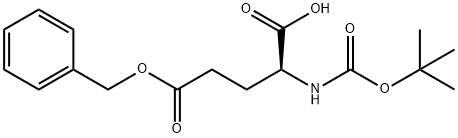
- Chemical Name:Boc-L-Glutamic acid 5-benzylester
- CAS:13574-13-5
- MF:C17H23NO6
- Structure:
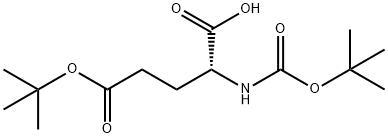
- Chemical Name:BOC-D-GLU(OTBU)-OH
- CAS:104719-63-3
- MF:C14H25NO6
- Structure:
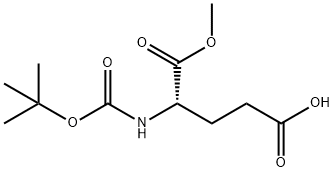
- Chemical Name:BOC-GLU-OME
- CAS:72086-72-7
- MF:C11H19NO6
- Structure:
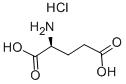
- Chemical Name:L-(+)-Glutamic acid hydrochloride
- CAS:138-15-8
- MF:C5H10ClNO4
- Structure:
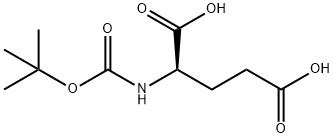
- Chemical Name:BOC-D-GLU-OH
- CAS:34404-28-9
- MF:C10H17NO6
- Structure:
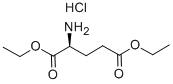
- Chemical Name:Diethyl L-glutamate hydrochloride
- CAS:1118-89-4
- MF:C9H18ClNO4
- Structure:
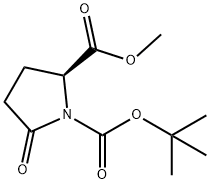
- Chemical Name:Boc-L-Pyroglutamic acid methyl ester
- CAS:108963-96-8
- MF:C11H17NO5
- Structure:
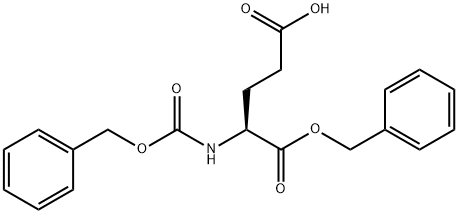
- Chemical Name:Cbz-L-Glutamic acid 1-benzyl ester
- CAS:3705-42-8
- MF:C20H21NO6
- Structure:
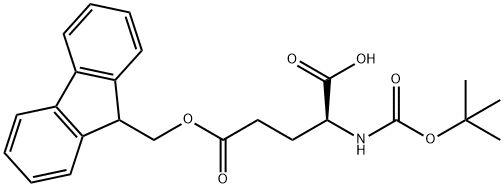
- Chemical Name:BOC-GLU(OFM)-OH
- CAS:123417-18-5
- MF:C24H27NO6
- Structure:
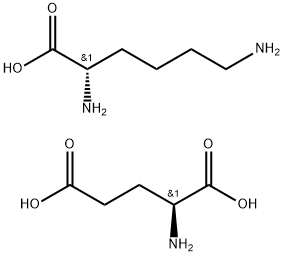
- Chemical Name:L-Lysine L-glutamate
- CAS:5408-52-6
- MF:C11H23N3O6
- Structure:
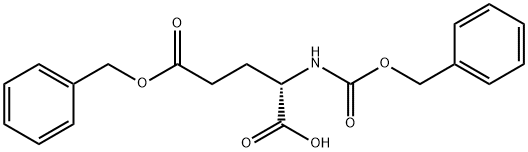
- Chemical Name:(S)-2-Benzyloxycarbonylamino-pentanedioic acid 5-benzyl ester
- CAS:5680-86-4
- MF:C20H21NO6
- Structure:
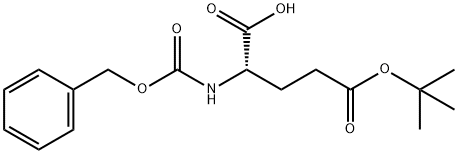
- Chemical Name:N-Cbz-L-Glutamic acid 5-tert-butyl ester
- CAS:3886-08-6
- MF:C17H23NO6
- Structure:
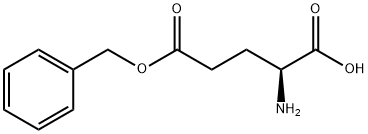
- Chemical Name:gamma-Benzyl L-glutamate
- CAS:1676-73-9
- MF:C12H15NO4
- Structure:
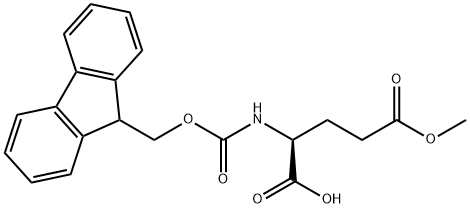
- Chemical Name:Fmoc-L-Glutamic acid gamma-methyl ester
- CAS:145038-50-2
- MF:C21H21NO6
- Structure:
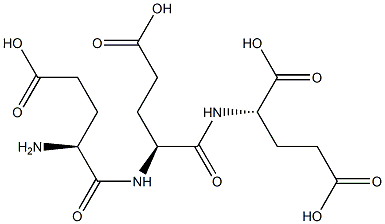
- Chemical Name:POLY-L-GLUTAMIC ACID SODIUM SALT
- CAS:26247-79-0
- MF:C15H23N3O10
- Structure:
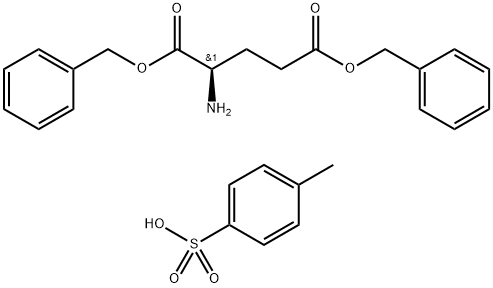
- Chemical Name:H-D-GLU(OBZL)-OBZL P-TOSYLATE
- CAS:19898-41-0
- MF:C26H29NO7S
- Structure:
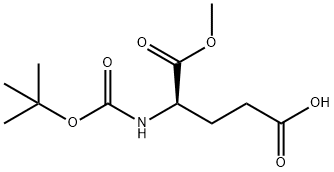
- Chemical Name:BOC-D-GLU-OME
- CAS:55227-00-4
- MF:C11H19NO6
- Chemical Name:EC 1.4.1.3
- CAS:9029-11-2
- MF:NULL
- Structure:
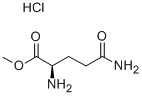
- Chemical Name:D-GLUTAMINE METHYL ESTER HYDROCHLORIDE
- CAS:74817-54-2
- MF:C6H13ClN2O3
- Structure:
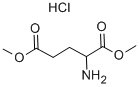
- Chemical Name:DIMETHYL DL-GLUTAMATE HYDROCHLORIDE
- CAS:13515-99-6
- MF:C7H14ClNO4
- Structure:
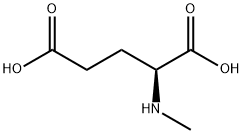
- Chemical Name:H-L-MEGLU-OH HCL
- CAS:6753-62-4
- MF:C6H11NO4
- Structure:
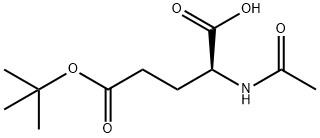
- Chemical Name:AC-GLU(OTBU)-OH
- CAS:84192-88-1
- MF:C11H19NO5
- Structure:
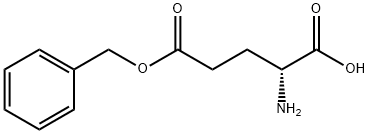
- Chemical Name:H-D-GLU(OBZL)-OH
- CAS:2578-33-8
- MF:C12H15NO4
- Structure:
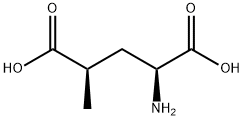
- Chemical Name:(2S,4R)-4-METHYLGLUTAMIC ACID
- CAS:31137-74-3
- MF:C6H11NO4
- Structure:
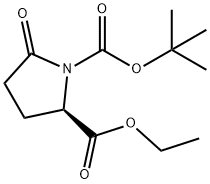
- Chemical Name:1-BOC-D-PYROGLUTAMIC ACID ETHYL ESTER
- CAS:144978-35-8
- MF:C12H19NO5
- Structure:
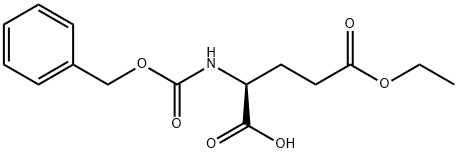
- Chemical Name:N-Cbz-L-glutamic acid 5-ethyl ester
- CAS:35726-62-6
- MF:C15H19NO6
- Structure:
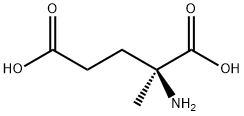
- Chemical Name:DL-2-METHYLGLUTAMIC ACID
- CAS:71-90-9
- MF:C6H11NO4
- Structure:
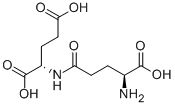
- Chemical Name:H-GAMMA-GLU-GLU-OH
- CAS:1116-22-9
- MF:C10H16N2O7
- Structure:
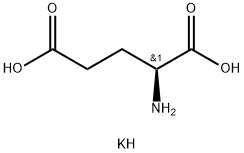
- Chemical Name:L-GLUTAMIC ACID MONOPOTASSIUM SALT
- CAS:19473-49-5
- MF:C5H10KNO4
- Structure:

- Chemical Name:4-FLUORO-DL-GLUTAMIC ACID
- CAS:2708-77-2
- MF:C5H8FNO4
- Structure:
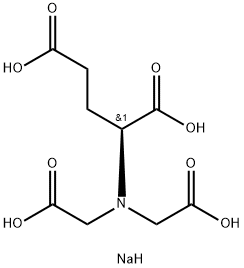
- Chemical Name:Tetrasodium glutamate diacetate
- CAS:51981-21-6
- MF:C9H14NNaO8
- Structure:
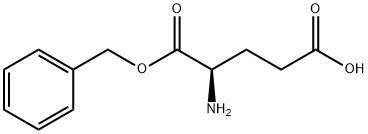
- Chemical Name:H-D-Glu-OBzl
- CAS:79338-14-0
- MF:C12H15NO4
- Structure:
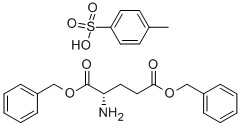
- Chemical Name:L-Glutamic acid dibenzyl ester tosylate
- CAS:2791-84-6
- MF:C26H29NO7S
- Structure:
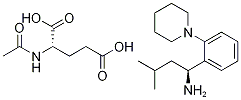
- Chemical Name:(S,S')-3-Methyl-1-(2-piperidinophenyl)butylamine, n-acetyl-glutamate salt
- CAS:219921-94-5
- MF:C23H37N3O5
- Structure:
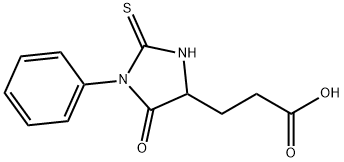
- Chemical Name:PTH-L-GLUTAMIC ACID
- CAS:5624-27-1
- MF:C12H12N2O3S
- Structure:
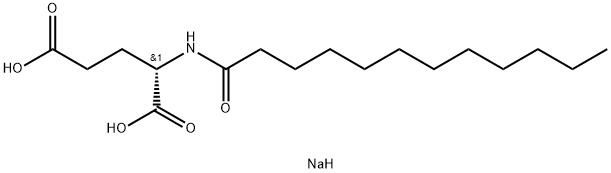
- Chemical Name:Sodium lauroyl glutamate
- CAS:29923-31-7
- MF:C17H32NNaO5
- Structure:
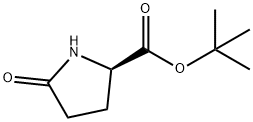
- Chemical Name:D-PYROGLUTAMIC ACID TERT-BUTYL ESTER
- CAS:205524-46-5
- MF:C9H15NO3
- Structure:
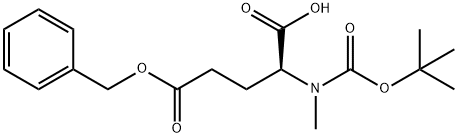
- Chemical Name:BOC-N-ME-GLU(OBZL)-OH
- CAS:200615-91-4
- MF:C18H25NO6
- Structure:
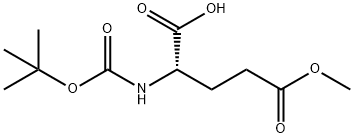
- Chemical Name:BOC-GLU(OME)-OH
- CAS:45214-91-3
- MF:C11H19NO6
- Structure:
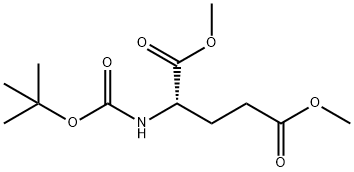
- Chemical Name:(R)-N-Boc-glutamic acid-1,5-dimethyl ester
- CAS:59279-60-6
- MF:C12H21NO6
- Structure:
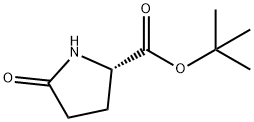
- Chemical Name:tert-butyl 5-oxo-L-prolinate
- CAS:35418-16-7
- MF:C9H15NO3
- Structure:
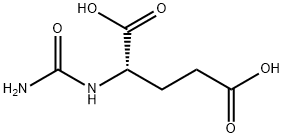
- Chemical Name:N-CARBAMYL-L-GLUTAMIC ACID
- CAS:1188-38-1
- MF:C6H10N2O5
- Structure:
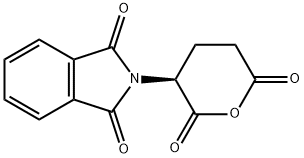
- Chemical Name:PHTHALOYL-L-GLUTAMIC ANHYDRIDE
- CAS:25830-77-7
- MF:C13H9NO5
- Structure:
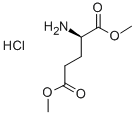
- Chemical Name:H-D-GLU(OME)-OME HCL
- CAS:27025-25-8
- MF:C7H14ClNO4
- Structure:
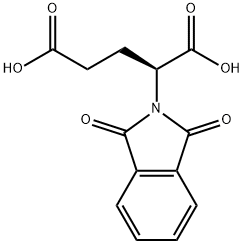
- Chemical Name:N-PHTHALOYL-L-GLUTAMIC ACID
- CAS:340-90-9
- MF:C13H11NO6
- Structure:

- Chemical Name:N-(4-AMINOBENZOYL)-L-GLUTAMIC ACID DIETHYL ESTER
- CAS:13726-52-8
- MF:C16H22N2O5
- Structure:
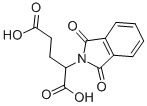
- Chemical Name:PHTHALYL-DL-GLUTAMIC ACID
- CAS:2301-52-2
- MF:C13H11NO6
- Structure:
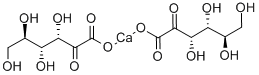
- Chemical Name:2-KETO-D-GLUCONIC ACID HEMICALCIUM SALT
- CAS:3470-37-9
- MF:C12H18CaO14
- Structure:
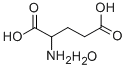
- Chemical Name:DL-Glutamic acid monohydrate
- CAS:19285-83-7
- MF:C5H11NO5
- Structure:
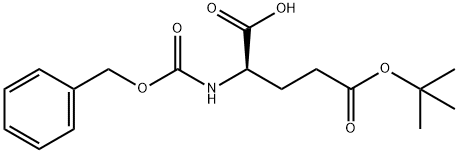
- Chemical Name:Z-D-GLU(OTBU)-OH
- CAS:51644-83-8
- MF:C17H23NO6
- Structure:
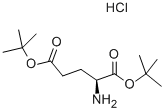
- Chemical Name:L-Glutamic acid di-tert-butyl ester hydrochloride
- CAS:32677-01-3
- MF:C13H26ClNO4
- Structure:
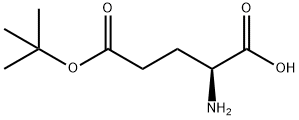
- Chemical Name:L-Glutamic acid 5-tert-butyl ester
- CAS:2419-56-9
- MF:C9H17NO4
- Structure:
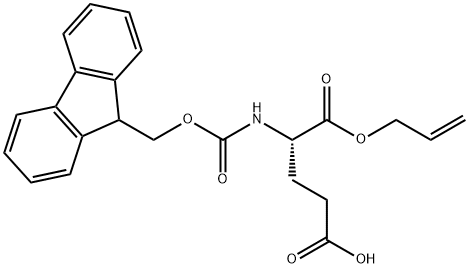
- Chemical Name:FMOC-GLU-OALL
- CAS:144120-54-7
- MF:C23H23NO6
- Structure:
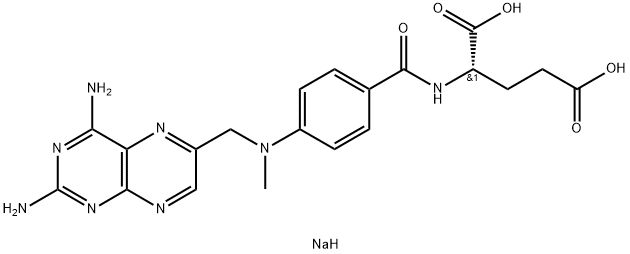
- Chemical Name:Methotrexate disodium salt
- CAS:7413-34-5
- MF:C20H23N8NaO5
- Structure:
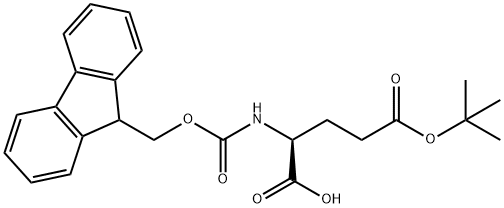
- Chemical Name:Fmoc-L-glutamic acid 5-tert-butyl ester
- CAS:71989-18-9
- MF:C24H27NO6
- Structure:
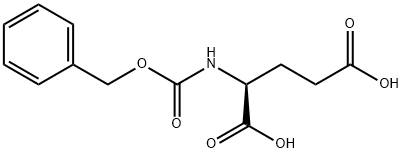
- Chemical Name:N-Cbz-L-glutamic acid
- CAS:1155-62-0
- MF:C13H15NO6
- Structure:
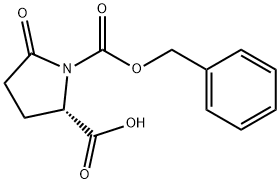
- Chemical Name:Z-PYR-OH
- CAS:32159-21-0
- MF:C13H13NO5
- Structure:
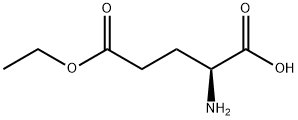
- Chemical Name:H-GLU(OET)-OH
- CAS:1119-33-1
- MF:C7H13NO4
- Structure:
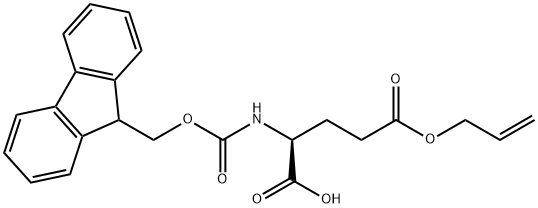
- Chemical Name:FMOC-GLU(OALL)-OH
- CAS:133464-46-7
- MF:C23H23NO6