Glutamic acid derivatives
Glutamic acid,Scientific name: α- aminoglutaric acid —so named because it is extracted from grains. α- aminoglutaric acid is widespread and almost can be found in all proteins. Glutamic acid is a protein hydrolysate, a colorless massive crystalline. It is soluble in water while insoluble in benzene, phenol and other organic solvent. Glutamic acid is an acidic amino acid because its aqueous solution is acidic. Under the physiological condition, glutamic acid unites with glycine and cysteine to form glutathione, a substance with important physiological function. Glutamic acid possesses umami, and its sodium salt is monosodium glutamate (MSG). MSG will decompose at high temperatures, and is vulnerable to great heat.
MSG is first discovered by Japanese chemist Ikeda. He discovered it by chance when he found the kelp soup containing a delicate flavour. On investigation, he has extracted 2g MSG from 100 kg kelp. A large number of MSG which called Ajinomoto have produced in Japan later. In 1930s, the Ajinomoto in China has produced by Shanghai TianChu Ajinomoto Factory. These products are exported to countries and receive the prestige. Hydrolyzing with natural gliadin or casein, adjusting pH to 3.3 to obtain glutamic acid precipitation, adding an appropriate amount of sodium hydroxide after separating can get MSG. MSG can also be synthesized but the cost is expensive, and they are usually still made by protein hydrolysis. After 1960s, Japanese use microbial fermentation to produce of glutamic acid. They put Curtobacterium, Chlorella, Bacillus into carbohydrate (mainly starch and glucose). Then they add urea, ammonium sulfate, ammonium chloride and an appropriate amount of biotin. These bacteria secrete large amounts of glutamic acid immediately to produce MSG. At present, China takes the method of fermentation to produce MSG. Glutamate can also combine with ammonia in the blood to form a nontoxic substance. So glutamate can also be used to treat diseases like hepatic coma and liver dysfunction during convalescence.
- Structure:
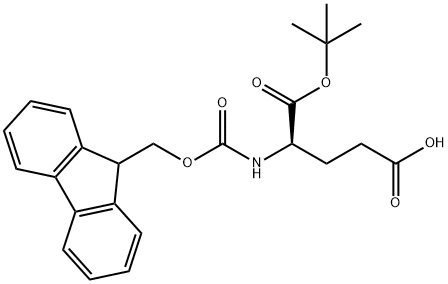
- Chemical Name:FMOC-D-GLU-OTBU
- CAS:109745-15-5
- MF:C24H27NO6
- Structure:
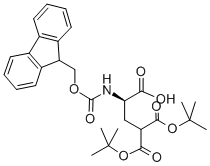
- Chemical Name:FMOC-D-GLA(OTBU)2-OH
- CAS:111662-65-8
- MF:C29H35NO8
- Structure:
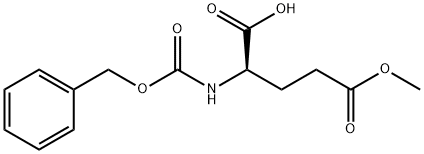
- Chemical Name:Z-D-GLU(OME)-OH
- CAS:27025-24-7
- MF:C14H17NO6
- Structure:
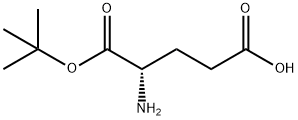
- Chemical Name:L-Glutamic acid α-tert·butyl ester
- CAS:45120-30-7
- MF:C9H17NO4
- Structure:
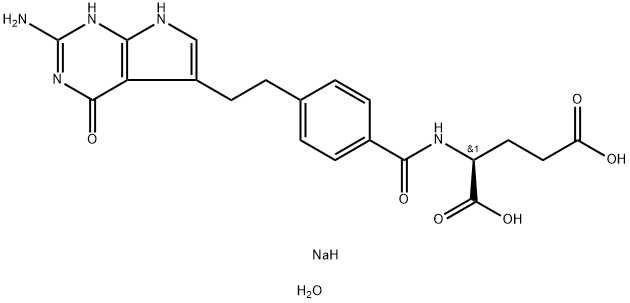
- Chemical Name:Pemetrexed disodium hemipentahydrate
- CAS:357166-30-4
- MF:C20H24N5NaO7
- Structure:
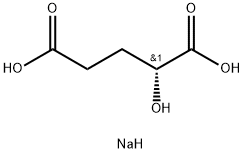
- Chemical Name:D-2-HYDROXYPENTANEDIOIC ACID DISODIUM SALT
- CAS:103404-90-6
- MF:C5H9NaO5
- Structure:
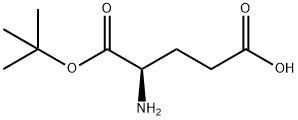
- Chemical Name:D-Glutamic acid 1-tert-butyl ester
- CAS:25456-76-2
- MF:C9H17NO4
- Structure:
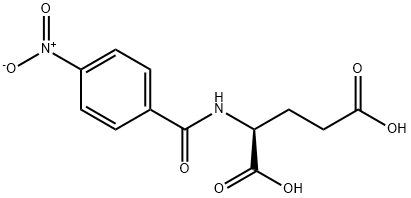
- Chemical Name:P-NITROBENZOYL-L-GLUTAMIC ACID
- CAS:6758-40-3
- MF:C12H12N2O7
- Structure:
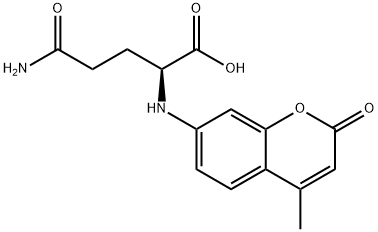
- Chemical Name:H-GLU(AMC)-OH
- CAS:72669-53-5
- MF:C15H16N2O5
- Structure:
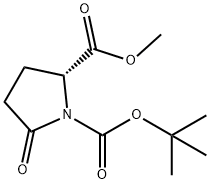
- Chemical Name:(R)-N-BOC-5-METHOXYCARBONYL-2-PYRROLIDINONE
- CAS:128811-48-3
- MF:C11H17NO5
- Structure:
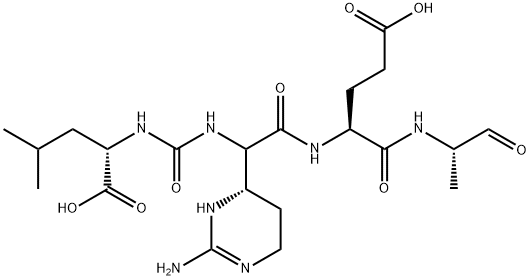
- Chemical Name:ELASTATINAL
- CAS:51798-45-9
- MF:C21H36N8O7
- Structure:
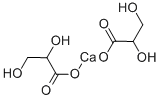
- Chemical Name:DL-GLYCERIC ACID HEMICALCIUM SALT HYDRATE
- CAS:67525-74-0
- MF:C6H10CaO8
- Structure:
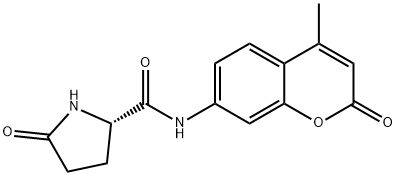
- Chemical Name:L-PYROGLUTAMIC ACID 4-METHYL-7-COUMARINYLAMIDE HYDRATE
- CAS:66642-36-2
- MF:C15H14N2O4
- Structure:
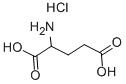
- Chemical Name:DL-GLUTAMIC ACID HYDROCHLORIDE
- CAS:15767-75-6
- MF:C5H10ClNO4
- Structure:
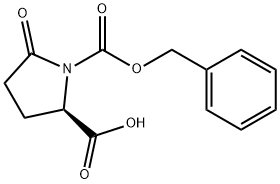
- Chemical Name:Z-D-PYR-OH
- CAS:78339-57-8
- MF:C13H13NO5
- Structure:
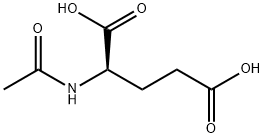
- Chemical Name:N-Acetyl-D-glutamic acid
- CAS:19146-55-5
- MF:C7H11NO5
- Structure:
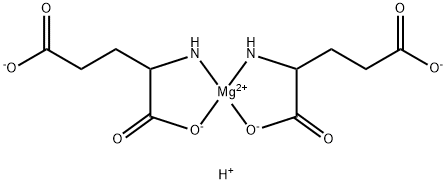
- Chemical Name:MAGNESIUM L-GLUTAMATE TETRAHYDRATE
- CAS:18543-68-5
- MF:C10H13MgN2O8-
- Structure:
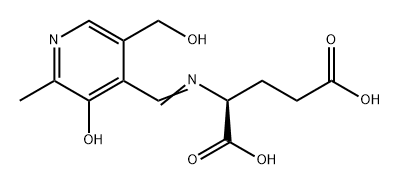
- Chemical Name:PYRIDOXYLIDENE-L-GLUTAMIC ACID DIPOTASSIUM SALT
- CAS:13934-03-7
- MF:C13H16N2O6
- Structure:
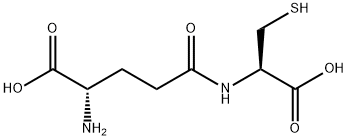
- Chemical Name:GAMMA-GLU-CYS TRIFLUOROACETATE SALT
- CAS:636-58-8
- MF:C8H14N2O5S
- Structure:
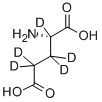
- Chemical Name:L-GLUTAMIC-2,3,3,4,4-D5 ACID
- CAS:2784-50-1
- MF:C5H4D5NO4
- Structure:
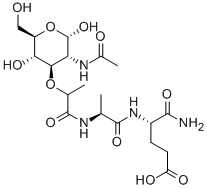
- Chemical Name:AC-MURAMYL-ALA-GLU-NH2
- CAS:59331-38-3
- MF:C19H32N4O11
- Structure:
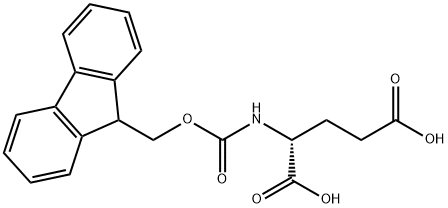
- Chemical Name:N-[(9H-FLUOREN-9-YLMETHOXY)CARBONYL]-D-GLUTAMIC ACID
- CAS:104091-09-0
- MF:C20H19NO6
- Structure:
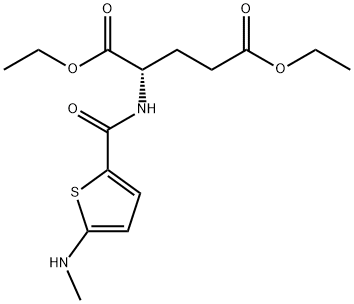
- Chemical Name:Diethyl N-[5-methylamino-2-thenoyl]-L-glutamate
- CAS:112889-02-8
- MF:C15H22N2O5S
- Structure:
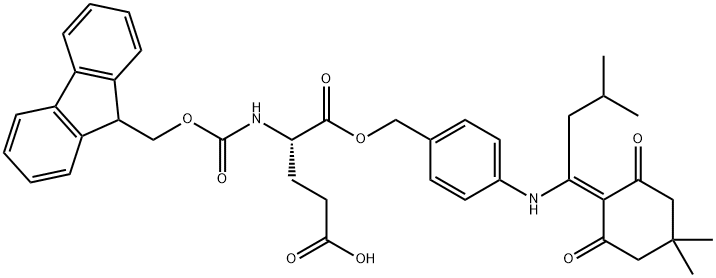
- Chemical Name:FMOC-GLU(ODMAB)-OH
- CAS:172611-75-5
- MF:C40H44N2O8
- Structure:
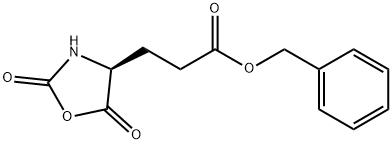
- Chemical Name:5-Benzyl L-glutamate N-carboxyanhydride
- CAS:3190-71-4
- MF:C13H13NO5
- Structure:
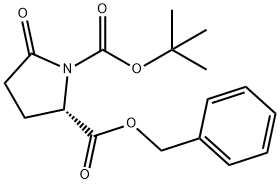
- Chemical Name:BOC-L-PYROGLUTAMIC ACID BENZYL ESTER
- CAS:113400-36-5
- MF:C17H21NO5
- Structure:
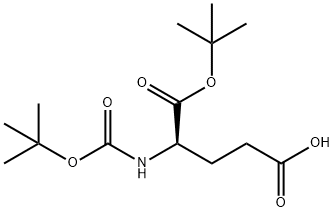
- Chemical Name:BOC-D-GLU-OTBU
- CAS:73872-71-6
- MF:C14H25NO6
- Structure:
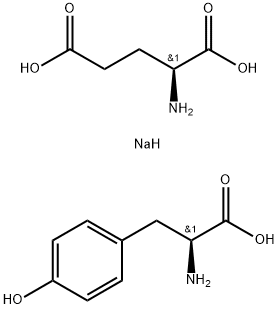
- Chemical Name:POLY(GLU, TYR) SODIUM SALT
- CAS:97105-00-5
- MF:C14H21N2NaO7
- Structure:
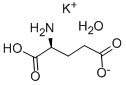
- Chemical Name:L-GLUTAMIC ACID MONOPOTASSIUM SALT
- CAS:6382-01-0
- MF:C5H8NO4.K.H2O
- Structure:
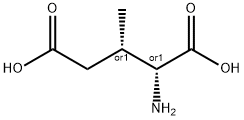
- Chemical Name:(+/-)-THREO-3-METHYLGLUTAMIC ACID
- CAS:63088-04-0
- MF:C6H11NO4
- Structure:
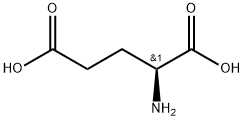
- Chemical Name:Polyglutamic acid
- CAS:25513-46-6
- MF:C5H9NO4
- Structure:
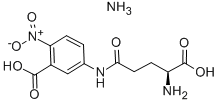
- Chemical Name:L-GLUTAMIC ACID GAMMA-(3-CARBOXY-4-NITROANILIDE) AMMONIUM SALT
- CAS:63699-78-5
- MF:C12H16N4O7
- Structure:
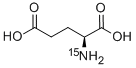
- Chemical Name:L-GLUTAMIC-15N ACID
- CAS:21160-87-2
- MF:C5H9NO4
- Structure:
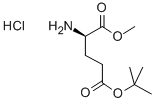
- Chemical Name:H-D-Glu(Otbu)-OMe.HCL
- CAS:16948-36-0
- MF:C10H19NO4.ClH
- Structure:
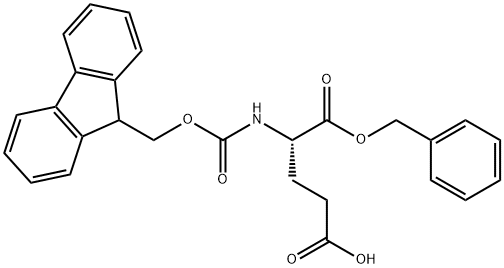
- Chemical Name:FMOC-GLU-OBZL
- CAS:122350-52-1
- MF:C27H25NO6
- Structure:
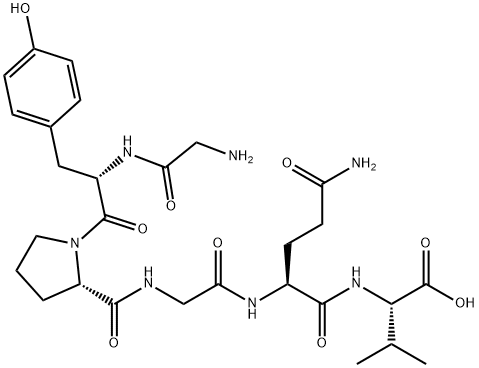
- Chemical Name:H-GLY-TYR-PRO-GLY-GLN-VAL-OH
- CAS:225779-44-2
- MF:C28H41N7O9
- Structure:
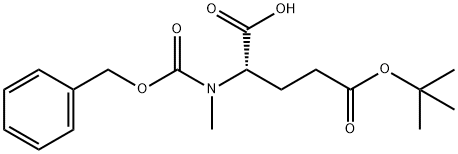
- Chemical Name:Z-N-ME-GLU(OTBU)-OH
- CAS:42417-71-0
- MF:C18H25NO6
- Structure:
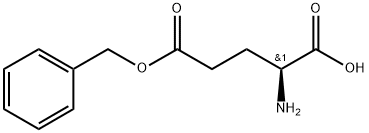
- Chemical Name:POLY-GAMMA-BENZYL L-GLUTAMATE
- CAS:25014-27-1
- MF:C12H15NO4
- Structure:
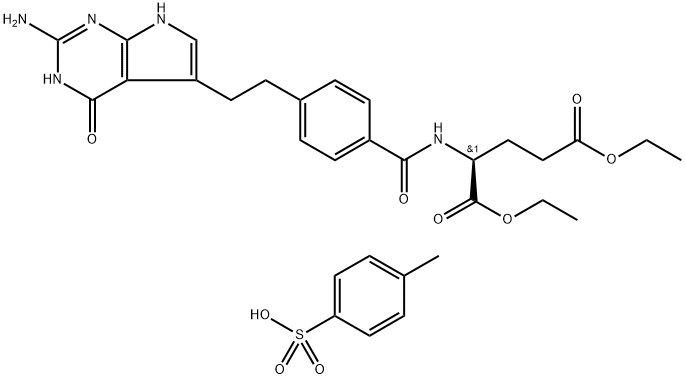
- Chemical Name:N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid 1,5-diethyl ester 4-methylbenzenesulfonate
- CAS:165049-28-5
- MF:C31H37N5O9S
- Structure:
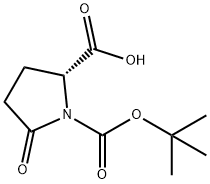
- Chemical Name:BOC-D-PYR-OH
- CAS:160347-90-0
- MF:C10H15NO5
- Structure:
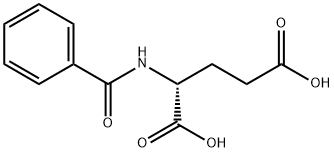
- Chemical Name:(+)-N-BENZOYLGLUTAMIC ACID
- CAS:58094-18-1
- MF:C12H13NO5
- Structure:
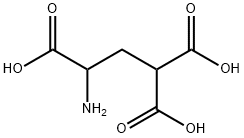
- Chemical Name:H-DL-GLA-OH
- CAS:56271-99-9
- MF:C6H9NO6
- Structure:
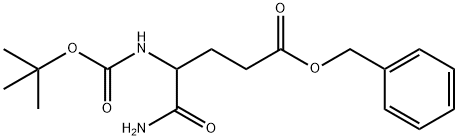
- Chemical Name:BENZYL 5-AMINO-4-[(TERT-BUTOXYCARBONYL)AMINO]-5-OXOPENTANOATE
- CAS:292870-04-3
- MF:C17H24N2O5
- Chemical Name:POLY-L-PHENYLALANINE
- CAS:25191-15-5
- MF:
- Structure:

- Chemical Name:POTASSIUM L-GLUTAMATE MONOHYDRATE
- CAS:
- MF:C5H10KNO5
- Structure:
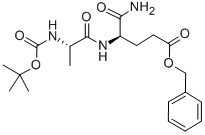
- Chemical Name:BOC-ALA-D-GLU(OBZL)-NH2
- CAS:18814-49-8
- MF:C20H29N3O6
- Structure:
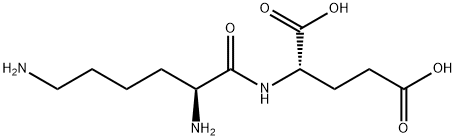
- Chemical Name:H-LYS-GLU-OH
- CAS:45234-02-4
- MF:C11H21N3O5
- Structure:
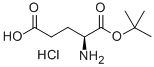
- Chemical Name:L-Glutamic acid 1-tert-Butyl ester hydrochloride
- CAS:144313-55-3
- MF:C9H18ClNO4
- Structure:
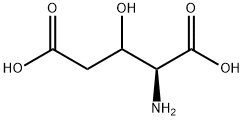
- Chemical Name:3-hydroxyglutamic acid
- CAS:533-62-0
- MF:C5H9NO5
- Structure:
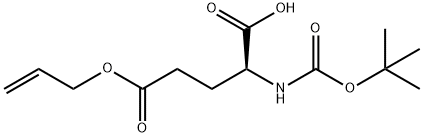
- Chemical Name:Boc-L-glutamic acid γ-allyl ester
- CAS:132286-79-4
- MF:C13H21NO6
- Structure:
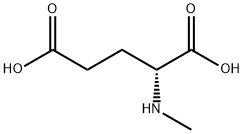
- Chemical Name:N-Methyl-D-glutamic acid
- CAS:77481-28-8
- MF:C6H11NO4
- Structure:
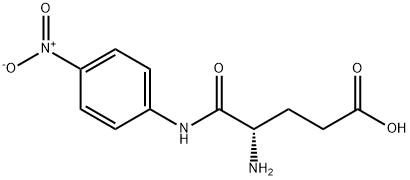
- Chemical Name:H-GLU-PNA
- CAS:24032-35-7
- MF:C11H13N3O5
- Structure:
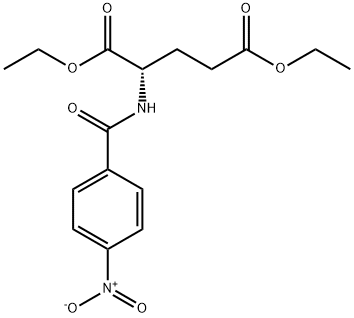
- Chemical Name:N-(4-NITROBENZOYL)-L-GLUTAMIC ACID DIETHYL ESTER
- CAS:7148-24-5
- MF:C16H20N2O7
- Structure:
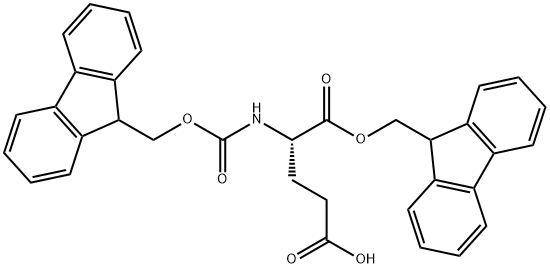
- Chemical Name:FMOC-GLU-OFM
- CAS:200616-18-8
- MF:C34H29NO6
- Structure:
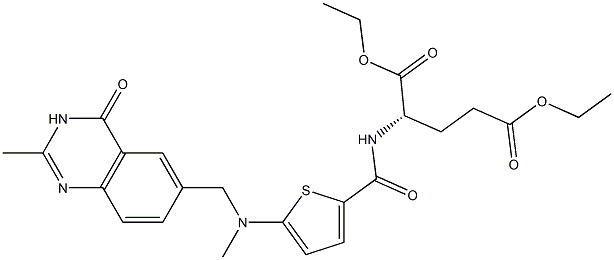
- Chemical Name:Diethyl N-[5-[N-[(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]-N-methylamino]-2-thenoyl]-L-glutamate
- CAS:132463-02-6
- MF:C25H30N4O6S
- Structure:
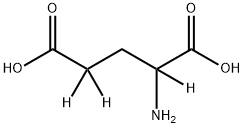
- Chemical Name:DL-GLUTAMIC-2,4,4-D3 ACID
- CAS:96927-56-9
- MF:C5H9NO4
- Chemical Name:DL-Glu(OBzl)-OBzl Tosoh
- CAS:
- MF:C19H21NO4·C7H8O3S
- Structure:
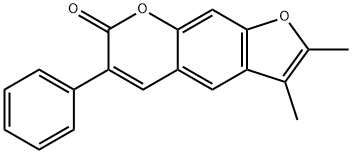
- Chemical Name:TOS-GLU-OH
- CAS:4216-80-2
- MF:C19H14O3
- Structure:
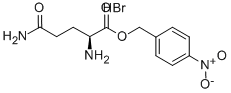
- Chemical Name:H-GLN-ONB HBR
- CAS:14349-18-9
- MF:C12H16BrN3O5
- Structure:
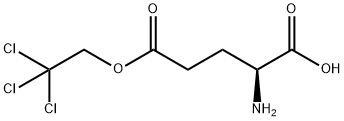
- Chemical Name:L-GLUTAMIC ACID GAMMA-(2,2,2-TRICHLOROETHYL) ESTER
- CAS:92739-23-6
- MF:C7H10Cl3NO4
- Structure:
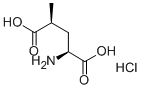
- Chemical Name:(2S,4S)-4-METHYLGLUTAMIC ACID HYDROCHLORIDE
- CAS:160806-12-2
- MF:C6H11NO4.HCl
- Structure:
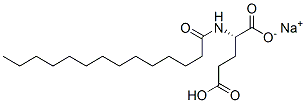
- Chemical Name:sodium hydrogen N-(1-oxotetradecyl)-L-glutamate
- CAS:38517-37-2
- MF:C19H34NNaO5
- Structure:
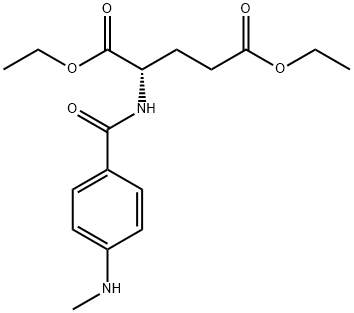
- Chemical Name:diethyl N-[4-(methylamino)benzoyl]-L-glutamate
- CAS:2378-95-2
- MF:C17H24N2O5
- Structure:
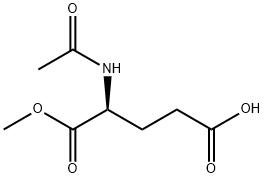
- Chemical Name:AC-GLU-OME
- CAS:17015-15-5
- MF:C8H13NO5
- Structure:
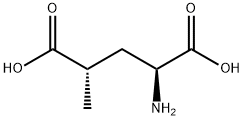
- Chemical Name:(2S,4S)-4-METHYLGLUTAMIC ACID
- CAS:6141-27-1
- MF:C6H11NO4
- Structure:
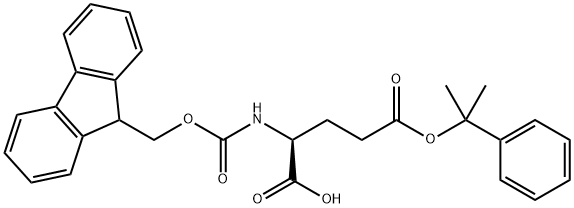
- Chemical Name:FMOC-GLU(O-2-PHIPR)-OH
- CAS:200616-39-3
- MF:C29H29NO6
- Structure:
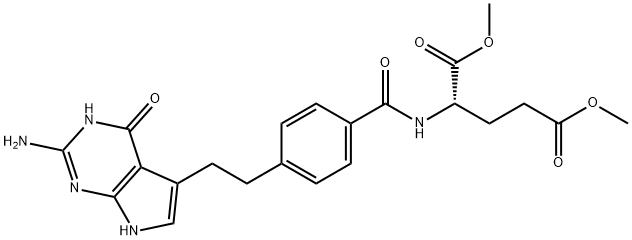
- Chemical Name:N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid 1,5-dimethyl ester
- CAS:155405-81-5
- MF:C22H25N5O6
- Structure:
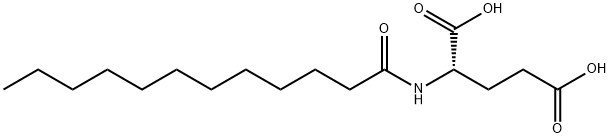
- Chemical Name:N-LAUROYL-L-GLUTAMIC ACID
- CAS:3397-65-7
- MF:C17H31NO5
- Structure:
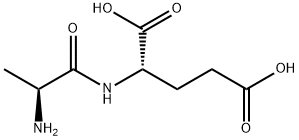
- Chemical Name:H-ALA-GLU-OH
- CAS:13187-90-1
- MF:C8H14N2O5
- Structure:
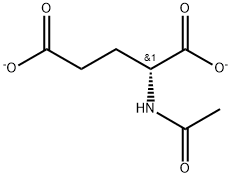
- Chemical Name:N-Acetyl-D-glutamic acid
- CAS:339072-10-5
- MF:C7H9NO5(-2)
- Structure:
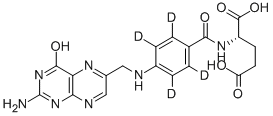
- Chemical Name:Folic Acid-D4
- CAS:171777-72-3
- MF:C19H15D4N7O6
- Structure:
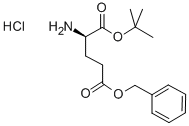
- Chemical Name:H-D-GLU(OBZL)-OTBU HCL
- CAS:90159-60-7
- MF:C16H24ClNO4
- Structure:
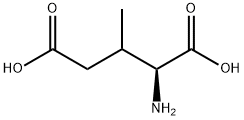
- Chemical Name:(2S,3R)-3-METHYLGLUTAMIC ACID HYDROCHLORIDE SALT
- CAS:2445-97-8
- MF:C6H11NO4
- Chemical Name:Fmoc-Glu-OtBu
- CAS:4793-07-7
- MF:C24H27NO6
- Structure:
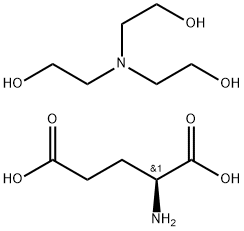
- Chemical Name:l-Glutamic acid, N-coco acyl derivs., compds. with triethanolamine (1:1)
- CAS:68187-29-1
- MF:C11H24N2O7
- Chemical Name:l-Glutamic acid, N-coco acyl derivs., monosodium salts
- CAS:68187-32-6
- MF:C5H9NO4?Na
- Structure:
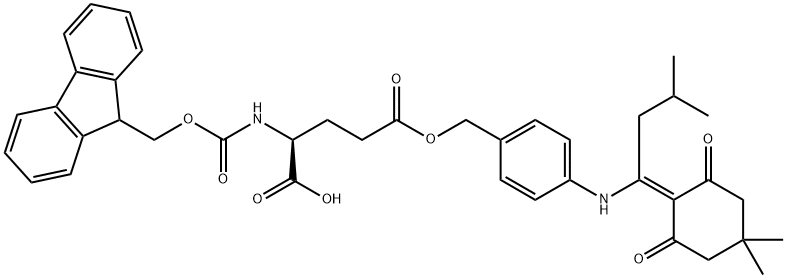
- Chemical Name:FMOC-GLU(ODMAB)-OH
- CAS:268730-86-5
- MF:C40H44N2O8
- Structure:
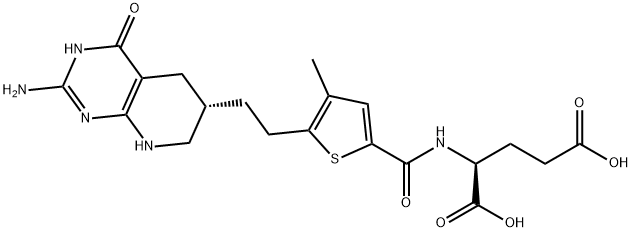
- Chemical Name:N-((5-(2-((6S)-2-Amino-1,4,5,6,7,8-hexahydro-4-oxopyrido[2,3-d]pyrimidin-6-yl)ethyl)-4-methyl-2-thienyl)carbonyl)-L-glutamic acid
- CAS:446022-33-9
- MF:C20H25N5O6S
- Structure:
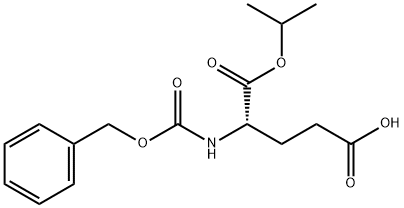
- Chemical Name:L-Glutamic acid, N-[(phenylmethoxy)carbonyl]-, 1-1-methylethyl) ester
- CAS:88815-54-7
- MF:C16H21NO6
- Structure:
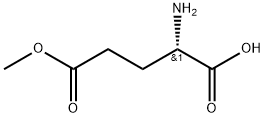
- Chemical Name:POLY(GAMMA-METHYL L-GLUTAMATE)
- CAS:25086-16-2
- MF:C6H11NO4
- Structure:
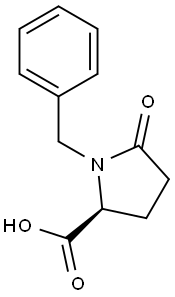
- Chemical Name:(S)-1-BENZYL-5-CARBOXY-2-PYRROLIDINONE
- CAS:7535-59-3
- MF:C12H13NO3
- Chemical Name:Glutamate dehydrogenase from bovine liver
- CAS:
- MF:
- Chemical Name:SODIUM HYDROGENATED TALLOWOYL GLUTAMATE
- CAS:
- MF:
- Structure:
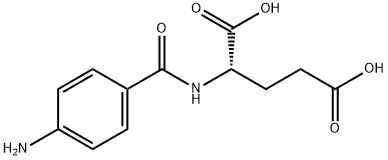
- Chemical Name:N-(4-Aminobenzoyl)-DL-glutamic acid
- CAS:4230-33-5
- MF:C12H14N2O5
- Chemical Name:l-Glutamic acid, N-coco acyl derivs., disodium salts
- CAS:68187-30-4
- MF:C5H7NNa2O4
- Structure:
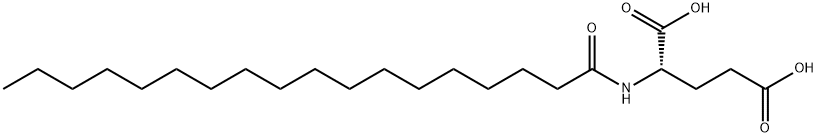
- Chemical Name:N-(1-oxooctadecyl)-L-glutamic acid
- CAS:3397-16-8
- MF:C23H43NO5
- Structure:

- Chemical Name:N-2,4-DNP-L-glutamic acid
- CAS:
- MF:C11H11N3O8
- Structure:

- Chemical Name:L-Glutamic acid 5-(3-carboxy-4-nitroanilide)ammonium salt
- CAS:
- MF:C19H23N7O12
- Structure:

- Chemical Name:D-Glutamic Acid diethyl ester hydrochloride
- CAS:1001-19-0
- MF:C9H18ClNO4
- Structure:

- Chemical Name:DIOCTYLDODECYL STEAROYL GLUTAMATE
- CAS:
- MF:C51H99NO5
- Structure:
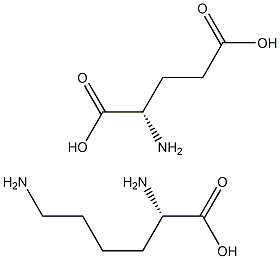
- Chemical Name:L-Lysine-L-glutamic acid
- CAS:
- MF:C11H23N3O6
- Chemical Name:Sodium cocoanutylglutamate
- CAS:
- MF:
- Chemical Name:Fmoc-D-Glu-OtBu
- CAS:
- MF:
- Structure:
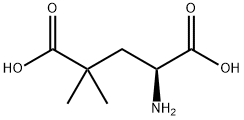
- Chemical Name:4-DIMETHYL-L-GLUTAMIC ACID
- CAS:151139-88-7
- MF:C7H13NO4
- Structure:
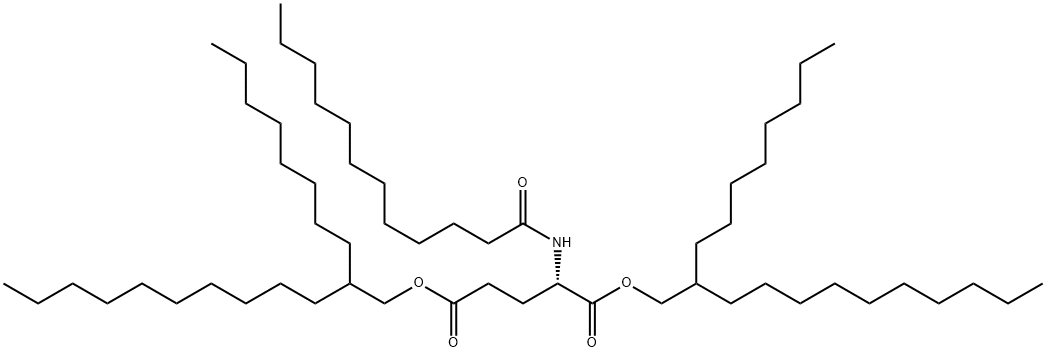
- Chemical Name:bis(2-octyldodecyl) N-(1-oxododecyl)-L-glutamate
- CAS:82204-94-2
- MF:C57H111NO5
- Structure:

- Chemical Name:3-Hydroxyglutamic acid hydrochloride
- CAS:
- MF:C5H10ClNO5
- Chemical Name:NR2B (Glutamate receptor)
- CAS:
- MF:
- Chemical Name:Anti-GAD-65 (glutamate decarboxylase)
- CAS:
- MF:
- Chemical Name:DIOCTYLDODECETH-2 LAUROYL GLUTAMATE
- CAS:
- MF:
- Structure:
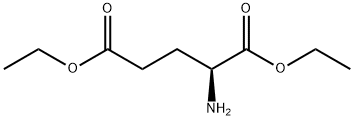
- Chemical Name:DIETHYL GLUTAMATE
- CAS:16450-41-2
- MF:C9H17NO4