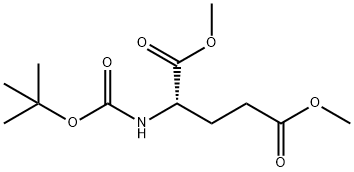
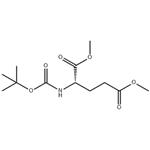
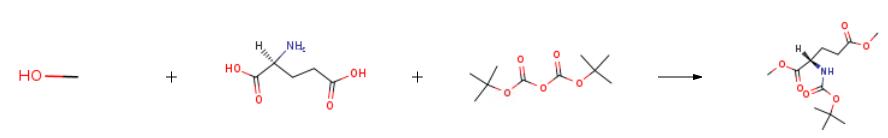(R)-N-Boc-glutamic acid-1,5-dimethyl ester can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development and chemical production processes.
L-glutamic acid (10.0 g, 67.97 mmol) was dissolved in 200 mL of methanol, and SOCl2(10.85 mL, 149.53 mmol)was slowly added dropwise under an ice bath. After the addition, reflux was performed at 65 C. After 2 hours, the reaction system was cooled to room temperature, and methanol was evaporated to dryness under reduced pressure to obtain a colorless viscous substance.This colorless viscous substance was dissolved in 200 mL of THF, (Boc)2O (22.25 g, 101.95 mmol) was addedunder an ice bath, and triethylamine (14.13 mL, 101.95 mmol) was slowly added dropwise.After the addition was complete, the reaction was allowed to proceed at room temperature overnight.After completion of the reaction, the solvent was distilled off under reduced pressure.The residue was dissolved in 200 mL of dichloromethane.The organic phase was washed with water (200 mL * 2), saturated citric acid solution (200 mL * 2), saturated sodium bicarbonate solution (200 mL * 2), and saturated brine (200 mL * 2) in that order.The organic phase was dried over anhydrous Na2SO4. Thefiltrate was collected by filtration, and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (petroleum ether: ethyl acetate = 4: 1 v / v) to give a colorless oily product (R)-N-Boc-glutamic acid-1,5-dimethyl ester (18.34 g, yield 98%)
[1] Angewandte Chemie - International Edition, 2014, vol. 53, # 28, p. 7335 - 7338
[2] Angew. Chem., 2014, vol. 126, # 28, p. 7463 - 7466,4
[3] Journal of Organic Chemistry, 2006, vol. 71, # 21, p. 8283 - 8286
[4] Tetrahedron Letters, 2004, vol. 45, # 37, p. 6963 - 6965
[5] Patent: US2012/172402, 2012, A1. Location in patent: Page/Page column 14-15