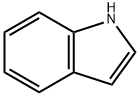
Indol
Bezeichnung:Indol
CAS-Nr120-72-9
Englisch Name:Indole
CBNumberCB8275772
SummenformelC8H7N
Molgewicht117.15
MOL-Datei120-72-9.mol
Synonyma
Indol
Indol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 51-54 °C (lit.) |
| Siedepunkt | 253-254 °C (lit.) |
| Dichte | 1.22 |
| chüttdichte | 230kg/m3 |
| Dampfdruck | 0.016 hPa (25 °C) |
| Brechungsindex | 1.6300 |
| FEMA | 2593 | INDOLE |
| Flammpunkt | >230 °F |
| storage temp. | 2-8°C |
| Löslichkeit | methanol: 0.1 g/mL, clear |
| pka | 3.17 (quoted, Sangster, 1989) |
| Aggregatzustand | Crystalline Powder |
| Farbe | White to slightly pink |
| PH | 5.9 (1000g/l, H2O, 20℃) |
| Geruch (Odor) | fecal odor, floral in high dilution |
| Odor Threshold | 0.0003ppm |
| Geruchsart | animal |
| Biologische Quelle | synthetic |
| Kennzeichnung gefährlicher | Xn,N,T |
| R-Sätze: | 21/22-37/38-41-50/53-36-39/23/24/25-23/24/25-52/53 |
| S-Sätze: | 26-36/37/39-60-61-45-36/37 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 1 |
| RTECS-Nr. | NL2450000 |
| F | 8-13 |
| TSCA | Yes |
| HS Code | 2933 99 20 |
| HazardClass | 9 |
| PackingGroup | III |
| Giftige Stoffe Daten | 120-72-9(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 1 g/kg (Smyth) |


