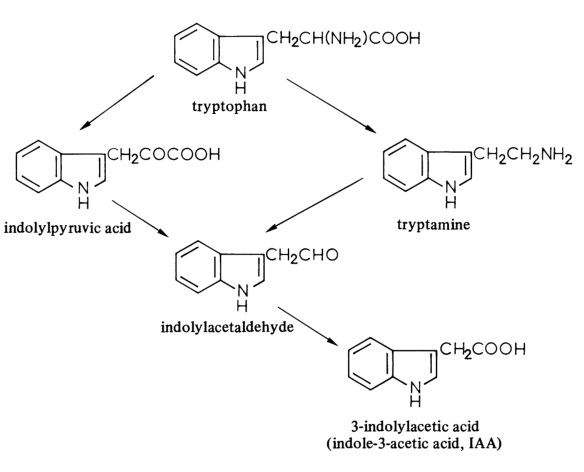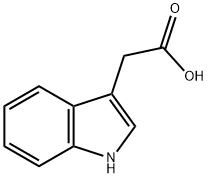
Indol-3-ylessigsure
Bezeichnung:Indol-3-ylessigsure
CAS-Nr87-51-4
Englisch Name:Indole-3-acetic acid
CBNumberCB8783347
SummenformelC10H9NO2
Molgewicht175.18
MOL-Datei87-51-4.mol
Synonyma
Indol-3-ylessigsure
Indol-3-ylessigsure physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 165-169 °C (lit.) |
| Siedepunkt | 306.47°C (rough estimate) |
| Dichte | 1.1999 (rough estimate) |
| chüttdichte | 620kg/m3 |
| Brechungsindex | 1.5460 (estimate) |
| Flammpunkt | 171°C |
| storage temp. | -20°C |
| Löslichkeit | DMSO:30.0(Max Conc. mg/mL);171.25(Max Conc. mM) |
| Aggregatzustand | crystalline |
| pka | 4.75(at 25℃) |
| Farbe | off-white to tan |
| Wasserlöslichkeit | Soluble in ethanol (50 mg/ml), methanol, DMSO, and chloroform (sparingly). Insoluble in water. |
| Decomposition | 167 ºC |
| Sensitive | Light Sensitive |
| Merck | 14,4964 |
| BRN | 143358 |
| Stabilität | Stable. Incompatible with strong oxidizing agents. Light sensitive. |
| LogP | 1.410 |
| Kennzeichnung gefährlicher | Xi |
| R-Sätze: | 36/37/38 |
| S-Sätze: | 22-24/25 |
| WGK Germany | 3 |
| RTECS-Nr. | NL3150000 |
| F | 8-10-23 |
| Hazard Note | Irritant |
| TSCA | Yes |
| HS Code | 29339990 |
| Toxizität | LD50 intraperitoneal in mouse: 150mg/kg |