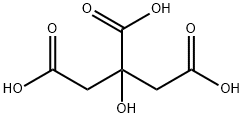
Zitronensure
Bezeichnung:Zitronensure
CAS-Nr77-92-9
Englisch Name:Citric acid
CBNumberCB9854361
SummenformelC6H8O7
Molgewicht192.12
MOL-Datei77-92-9.mol
Synonyma
Zitronensure
2-Hydroxy-1,2,3-propantricarbons?ure
beta-Hydroxytricarballyls?ure
Zitronensure physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 153-159 °C (lit.) |
| Siedepunkt | 248.08°C (rough estimate) |
| Dichte | 1.67 g/cm3 at 20 °C |
| chüttdichte | 560kg/m3 |
| Dampfdichte | 7.26 (vs air) |
| Dampfdruck | <0.1 hPa (20 °C) |
| Brechungsindex | 1.493~1.509 |
| FEMA | 2306 | CITRIC ACID |
| Flammpunkt | 100 °C |
| storage temp. | 2-8°C |
| Löslichkeit | Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15 °C. |
| Aggregatzustand | grit |
| pka | 3.14(at 20℃) |
| Farbe | White |
| Geruch (Odor) | Odorless |
| PH | 3.24(1 mM solution);2.62(10 mM solution);2.08(100 mM solution); |
| Biologische Quelle | synthetic |
| Geruchsart | odorless |
| Kennzeichnung gefährlicher | Xi,C,T |
| R-Sätze: | 41-36/37/38-36/38-37/38-34-36-35-61-60 |
| S-Sätze: | 26-39-37/39-24/25-36/37/39-45-36-53 |
| RIDADR | UN 1789 8/PG 3 |
| WGK Germany | 1 |
| RTECS-Nr. | GE7350000 |
| F | 9 |
| TSCA | Yes |
| HS Code | 2918 14 00 |
| Giftige Stoffe Daten | 77-92-9(Hazardous Substances Data) |
| Toxizität | LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen) |


