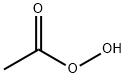
Peroxyessigsäure ...%
Bezeichnung:Peroxyessigsäure ...%
CAS-Nr79-21-0
Englisch Name:Peroxyacetic acid
CBNumberCB4399742
SummenformelC2H4O3
Molgewicht76.05
MOL-Datei79-21-0.mol
Synonyma
Peressigsure
Peroxyessigsäure ...%
Peroxyessigs?ure
Peroxyessigsäure ...% physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -44 °C |
| Siedepunkt | 105 °C |
| Dichte | 1.19 g/mL at 20 °C |
| Dampfdruck | Low |
| Brechungsindex | n |
| Flammpunkt | 41 °C |
| storage temp. | 2-8°C |
| pka | 8.2(at 25℃) |
| Aggregatzustand | liquid |
| Farbe | Colorless liquid |
| Geruch (Odor) | Acrid odor |
| PH | 1 (20°C in H2O) |
| Wasserlöslichkeit | soluble, >=10 g/100 mL at 19 ºC |
| Merck | 13,7229 |
| BRN | 1098464 |
| Stabilität | Unstable - may explode on heating. May react violently with organic materials. Incompatible with strong oxidizing agents, acetic anhydride, alkenes, organics. |
| LogP | -0.26 at 20℃ |
| CAS Datenbank | 79-21-0(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | O,C,N |
| R-Sätze: | 7-20/21/22-35-50-10-34-22-20 |
| S-Sätze: | 26-36/37/39-45-61-3/7-23-14A-14-60-9-7-3 |
| RIDADR | UN 3109 5.2 |
| WGK Germany | 2 |
| RTECS-Nr. | SD8750000 |
| F | 4.4-8 |
| Selbstentzündungstemperatur | Explodes when heated to 110 °C |
| HazardClass | 5.2 |
| PackingGroup | II |
| HS Code | 29159000 |
| Giftige Stoffe Daten | 79-21-0(Hazardous Substances Data) |
| Toxizität | LD50 (mg/kg) in rats: 1540 orally; in rabbits: 1410 dermally; LC50 in rats (mg/m3): 450 by inhalation (Klopotek) |




