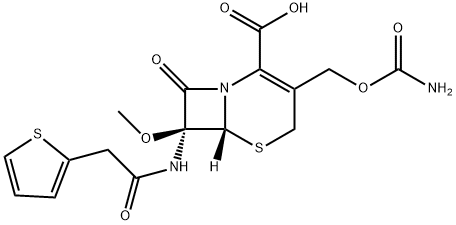
Цефокситин
- английское имяCefoxitin
- CAS №35607-66-0
- CBNumberCB9490581
- ФормулаC16H17N3O7S2
- мольный вес427.45
- EINECS252-641-2
- номер MDLMFCD00072014
- файл Mol35607-66-0.mol
химическое свойство
| Температура плавления | 149-150℃ |
| Температура кипения | 843℃ |
| плотность | 1.4441 (rough estimate) |
| показатель преломления | 1.6390 (estimate) |
| RTECS | XI0386500 |
| Fp | >110°(230°F) |
| температура хранения | 2-8°C |
| растворимость | DMSO (Slightly), Methanol (Slightly) |
| форма | Solid |
| пка | 2.2(at 25℃) |
| цвет | White to Off-White |
| Растворимость в воде | Predicted solubility in water is less than 0.2mg/ml |
| Справочник по базе данных CAS | 35607-66-0(CAS DataBase Reference) |
| FDA UNII | 6OEV9DX57Y |
| Словарь наркотиков NCI | Mefoxin |
| Код УВД | J01DC01 |
| Система регистрации веществ EPA | Cefoxitin (35607-66-0) |
| РИДАДР | 3077 |
| Класс опасности | 9 |
| Группа упаковки | III |
| кода HS | 30032013 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
Цефокситин химические свойства, назначение, производство
Описание
Cefoxitin contains the same C-7 side chain as cephalothin and the same C-3 side chain as cefuroxime. The most novel chemical feature of cefoxitin is the possession of an α-oriented methoxyl group in place of the normal H-atom at C-7. This increased steric bulk conveys very significant stability against β-lactamases. The inspiration for these functional groups was provided by the discovery of the naturally occurring antibiotic cephamycin C derived from fermentation of Streptomyces lactamdurans. Cephamycin C itself has not seen clinical use but, rather, has provided the structural clue that led to useful agents such as cefoxitin. Agents that contain this 7α methoxy group are commonly referred to as cephamycins. Ingenious chemical transformations now enable synthetic introduction of such a methoxy group into cephalosporins lacking this feature.Использование
Cefoxitin?is a semisynthetic, broad-spectrum second-generation cephalosporin with antibacterial activity. The activity of cefoxitin results in the weakening of the bacterial cell wall and causes cell lysis. Cefoxitin acts by interfering with cell wall synthesis. Its activity spectrum includes a broad range of gram-negative and gram-positive bacteria including anaerobes.Антимикробная активность
Most Gram-positive bacilli are susceptible, but L. monocytogenes is resistant. It is resistant to many Gramnegative β-lactamases and is active against organisms elaborating them, including some Citrobacter, Providencia, Serratia and Acinetobacter spp. Enterobacter spp. are resistant. It is moderately active against Bacteroides spp., but considerable strain variation in susceptibility occurs.Приобретенная устойчивость
Resistant strains of Bacteroides, some of which produce β-lactamases that hydrolyze cefoxitin, have been described. Resistance may be transferable to other Bacteroides spp. It is a potent inducer of chromosomal cephalosporinases of certain Gram-negative bacilli and can antagonize the effect of cefotaxime and other β-lactam agents.Клиническое использование
As for other group 3 cephalosporins, with particular emphasis on mixed infections including anaerobes, notably abdominal and pelvic sepsis. In considering its use, its low activity against aerobic Gram-positive cocci should be noted.Побочные эффекты
Reactions are those common to cephalosporins. Pain on intramuscular, and thrombophlebitis on intravenous, injection occur. Substantial changes can occur in the fecal flora, with virtual eradication of susceptible enterobacteria and non- fragilis Bacteroides, and appearance of, or increase in, yeasts, enterococci and other resistant bacteria including C. difficile. Development of meningitis due to H. influenzae and Str. pneumoniae in patients treated for other infections has been observed.Цефокситин запасные части и сырье
Цефокситин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 025-85710122 17714198479 | CHINA | 885 | 55 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8618032935937 18032916000 |
CHINA | 448 | 58 |