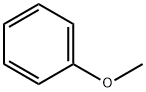
Anisole
- английское имяAnisole
- CAS №100-66-3
- CBNumberCB5100716
- ФормулаC7H8O
- мольный вес108.14
- EINECS202-876-1
- номер MDLMFCD00008354
- файл Mol100-66-3.mol
| Температура плавления | -37 °C (lit.) |
| Температура кипения | 154 °C (lit.) |
| плотность | 0.995 g/mL at 25 °C (lit.) |
| плотность пара | 3.7 (vs air) |
| давление пара | 10 mm Hg ( 42.2 °C) |
| показатель преломления | n |
| FEMA | 2097 | ANISOLE |
| Fp | 125 °F |
| температура хранения | Store below +30°C. |
| растворимость | 1.71g/l |
| форма | Liquid |
| цвет | Clear colorless |
| Относительная полярность | 0.198 |
| Запах | phenol, anise odor |
| Odor Type | phenolic |
| Биологические источники | synthetic |
| Пределы взрываемости | 0.34-6.3%(V) |
| Порог?обнаружения?запаха? | 0.057ppm |
| Растворимость в воде | 1.6 g/L (20 ºC) |
| Мерк | 14,669 |
| Номер JECFA | 1241 |
| БРН | 506892 |
| Диэлектрическая постоянная | 4.3(20℃) |
| Стабильность | Stable. Flammable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | RDOXTESZEPMUJZ-UHFFFAOYSA-N |
| LogP | 2.62 at 30℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | ANISOLE |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 100-66-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | B3W693GAZH |
| Справочник по химии NIST | Benzene, methoxy-(100-66-3) |
| Система регистрации веществ EPA | Anisole (100-66-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 10-38-20-36/37 | |||||||||
| Заявления о безопасности | 37/39-26-16-24/25 | |||||||||
| РИДАДР | UN 2222 3/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | BZ8050000 | |||||||||
| Температура самовоспламенения | 887 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29093090 | |||||||||
| Банк данных об опасных веществах | 100-66-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 3700 mg/kg (Taylor) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H336:Может вызывать сонливость или головокружение.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P242:Использовать неискрящие приборы.
P243:Принимать меры предосторожности против статических разрядов.
Anisole химические свойства, назначение, производство
Описание
Anisole is a colorless liquid with a spicy-sweet smell. Anisole can be produced by all the aforementioned methods. It is an important intermediate in the synthesis of organic compounds, for example fragrances and pharmaceuticals. The compound is also used as a solvent and as a heat transfer medium.Химические свойства
Anisole is a colorless to yellowish liquid with a characteristic pleasant, anise-like, agreeable, aromatic, spicy-sweet odor. It is used in perfumery.Вхождение
Anisole is a natural product found in apple juice and in the oil of Artemisia dracunculus var. turkestanica; also reported found in butter, Camembert cheese, roasted beef, olive (Olea europae), Malay apple, Jerusalem artichoke (Helianthus tuberosus), Bourbon vanilla, truffles, crab and sopadilla fruit (Achras sapota L.).Использование
Anisole is widely used as a solvent for the synthesis of various organic compounds, anethole, nonylphenol isomer 4-(3',6'-dimethyl-3-heptyl)phenol, perfumes, insect pheromones and pharmaceuticals. It finds application in the preparation of inorganic complexes and materials such as tin-core/tin oxide nanoparticles.прикладной
Anisole is an organic compound with the chemical formula C7H8O with a pleasant anise-like aroma, used in organic synthesis and also as a solvent, fragrance and insect repellent. For organic synthesis, it is also used as solvent, fragrance and insect repellent.Подготовка
Anisole is synthesized by reacting phenol and dimethyl sulfate in the presence of aqueous NaOH; by passing methyl chloride into a suspension of sodium phenolate in liquid ammonia.Определение
ChEBI: Anisole is a monomethoxybenzene that is benzene substituted by a methoxy group. It has a role as a plant metabolite.Общее описание
A clear straw-colored liquid with an aromatic odor. Insoluble in water and the same density as water. Vapors heavier than air. Flash point 125°F. Boiling point 307°F. Moderately toxic by ingestion. A skin irritant. Used to make perfumes, flavorings and as a solvent.Реакции воздуха и воды
Flammable. Ethers tend to form unstable peroxides when exposed to oxygen. Ethyl, isobutyl, ethyl tert-butyl, and ethyl tert-pentyl ether are particularly hazardous in this respect. Ether peroxides can sometimes be observed as clear crystals deposited on containers or along the surface of the liquid. Insoluble in waterПрофиль реактивности
Ethers, such as Anisole can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.Угроза здоровью
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.Профиль безопасности
Moderately toxic by ingestion and inhalation. A skin irritant. A flammable liquid. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid fumes.Возможный контакт
Anisole is used as a solvent; a flavoring, vermicide, making perfumes; and in organic synthesis.Методы очистки
Shake anisole with half its volume of 2M NaOH, and the emulsion is allowed to separate. Repeat three times, then wash twice with water, dry over CaCl2, filter, dry over sodium wire and finally distil it from fresh sodium under N2 using a Dean-Stark trap (samples in the trap being rejected until free from turbidity) [Caldin et al. J Chem Soc, Faraday Trans 1 72 1856 1976]. Alternatively dry it with CaSO4 or CaCl2, or by refluxing with sodium or BaO with crystalline FeSO4 or by passage through an alumina column. Traces of phenols are removed by prior shaking with 2M NaOH, followed by washing with water. It has been be purified by zone refining. [Beilstein 6 IV 548.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Anisole запасные части и сырье
сырьё
запасной предмет
- cleaner LS
- 4,5-Imidazoledicarboxylic кислота
- 6-METHOXY-1-NAPHTHOIC ACID
- 4-хлор-4'-метоксибензофенон
- 6-Hydroxy-1-naphthoic acid
- PYRIDIN-2-YLMETHYL-HYDRAZINE
- Fast Scarlet Base LG
- 3 - (4-Метоксибензоил) пропионовой кислоты
- Диаверидин
- 1-метилимидазол-4-карбоновой кислоты
- Метоксихлор
- Имидазол-4-метанол
- 4,5-ДИКАРБОКСИ-1-МЕТИЛ-1H-ИМИДАЗОЛ
- Cefcapene pivoxil
- PYRIDIN-3-YLMETHYL-HYDRAZINE
- Метилдопа
- 4,5-дицианоимидазол
- Верапамил
- 1H-имидазол-4-карбоновая кислота
- trisodium [5-acetamido-4-hydroxy-3-[[2-hydroxy-5-[[2-(sulphooxy)ethyl]sulphonyl]phenyl]azo]naphthalene-2,7-disulphonato(5-)]cuprate(3-)
- 1-ХЛОРМЕТИЛ-2,3,4-ТРИМЕТОКСИБЕНЗОЛ
- 1,5-диазабицикло[4.3.0]нон-5-ен
- Anisyl acetate
- 2-броманизол
- ПИРИДИН-4-ил-метил-ГИДРАЗИН
1of8
Anisole поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 571-88938639 +8617705817739 |
China | 52849 | 58 | ||
| +86-57-80696996 +86-19816093969 |
China | 16 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +8613223293093 | China | 80 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-0532-86867058 +86-18562606086 |
China | 478 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |